Figures & data
Table 1 Summary of patient demographics and baseline characteristics (ITT population)
Table 2 Summary and analysis of first and sustained CID in study A
Figure 1 Proportion of patients with a first and sustained CID (ITT population).
Notes: A moderate-to-severe exacerbation is defined as an acute worsening of COPD symptoms requiring the use of additional treatment including oral corticosteroids, antibiotics, emergency department treatment, or hospitalization.
Abbreviations: CID, clinically important deterioration; FEV1, forced expiratory volume in 1 second; ITT, intent-to-treat; SGRQ, St George’s Respiratory Questionnaire; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
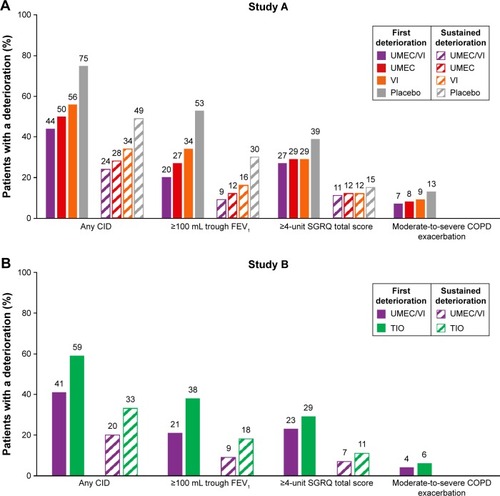
Figure 2 Time to first and sustained CID (ITT population).
Abbreviations: CID, clinically important deterioration; ITT, intent-to-treat; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
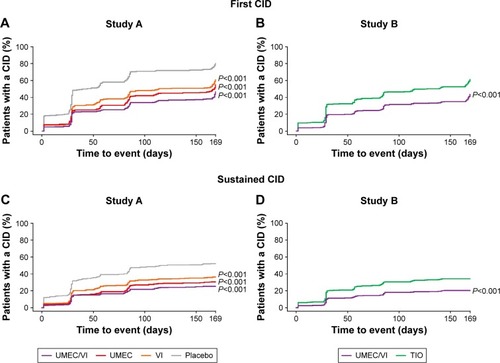
Table 3 Summary and analysis of first and sustained CID in Study B (ITT population)
Table S1 Summary of trough FEV1 at day 169 and SGRQ total score at day 168 in Study A (ITT population)
Table S2 Summary of trough FEV1 at day 169 and SGRQ total score at day 168 in Study B (ITT population)
Table S3 Summary of first and sustained composite CID by subgroup in Study A (ITT population)
Table S4 Summary of first and sustained composite CID by subgroup in Study B (ITT population)
