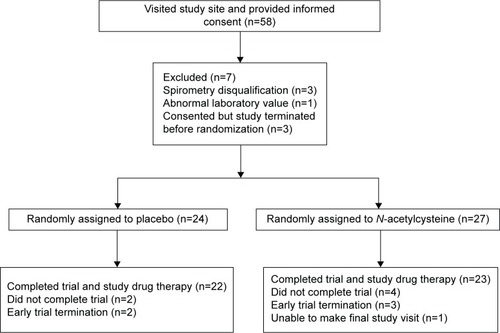
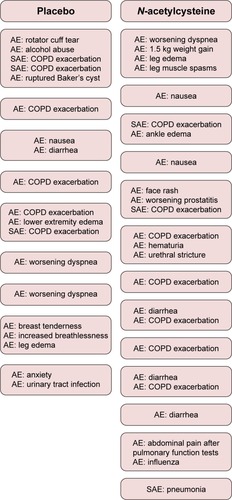
Figures & data
Table 1 Baseline characteristics
Table 2 Changes in survey scores over 8 weeks, according to treatment assignment
Table 3 Changes in lung function over 8 weeks, according to treatment assignment
Table 4 Changes in oxidation and inflammation biomarkers over 8 weeks, according to treatment assignment