Figures & data
Table 1 Demographics and clinical characteristics (ITT population)
Figure 1 Summary of patient disposition.
Abbreviations: TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
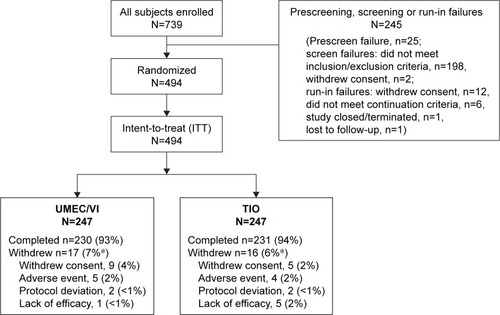
Table 2 Lung function outcomes (ITT population)
Figure 2 LS mean (SE) change from baseline in trough FEV1 (ITT population).
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 s; ITT, intent-to-treat; LS, least squares; SE, standard error; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
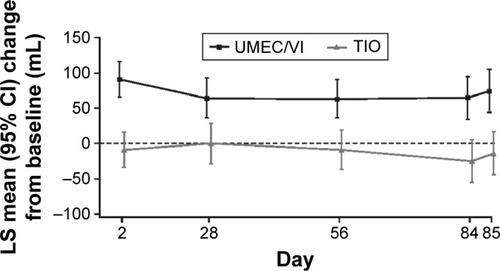
Figure 3 Serial LS mean change (95% CI) from baseline in FEV1 over 0–3 h on Day 1 (A) and Day 84 (B; ITT population).
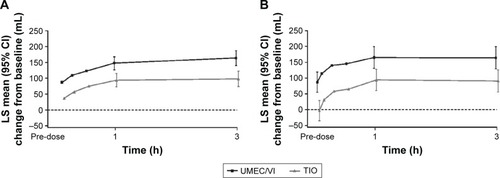
Figure 4 Serial LS mean change (95% CI) from baseline in FEV1 over 0–24 h on Day 1 (A) and Day 84 (B; 24-h population).
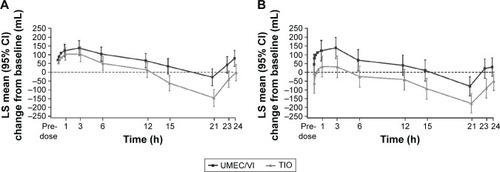
Table 3 Patient-reported outcomes (ITT population)
Figure 5 Responder analysis of clinically relevant change from baseline in breathlessness assessed by (A) TDI focal score, and health status assessed by either (B) SGRQ total score or (C) CAT score (ITT population).
Abbreviations: CAT, COPD Assessment Test; CI, confidence interval; ITT, intent-to-treat; OR, odds ratio; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
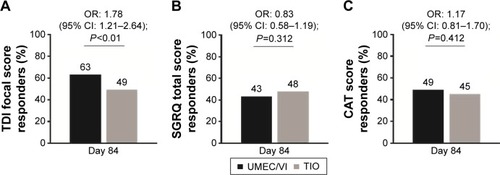
Table 4 Summary of AEs and COPD exacerbations (ITT population)
Figure S1 Serial LS mean change (95% CI) from baseline in FEV1 over 0–3 h on Day 28 (A) and Day 56 (B; ITT population).
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 s; ITT, intent-to-treat; LS, least squares; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
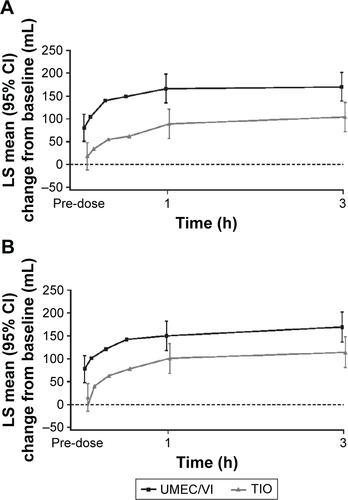
Table S1 Additional lung function outcomes (ITT population)
Table S2 Patient reported outcome responder analyses (ITT population)
