Figures & data
Figure 1 Patient flow diagram.
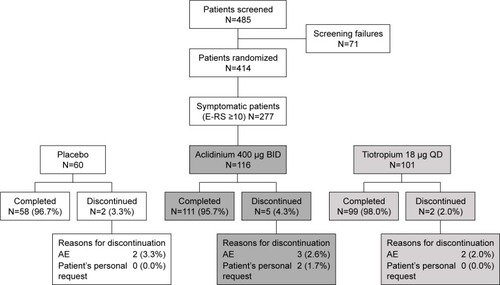
Table 1 Patient demographics in symptomatic patients (E-RS baseline score ≥10 units)
Figure 2 Symptomatic patients: mean changes from baseline in FEV1 at week 6 over 24 hours.
Abbreviations: FEV1, forced expiratory volume in 1 second; LS, least squares.
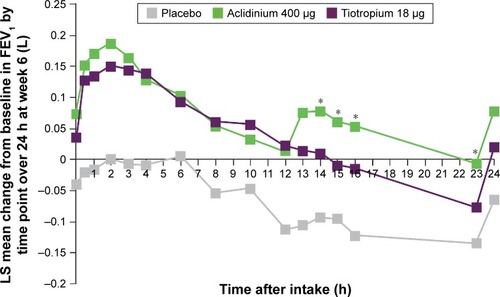
Figure 3 Symptomatic patients: change from baseline in trough FEV1 versus placebo at day 1 and week 6.
Abbreviations: FEV1, forced expiratory volume in 1 second; LS, least squares.
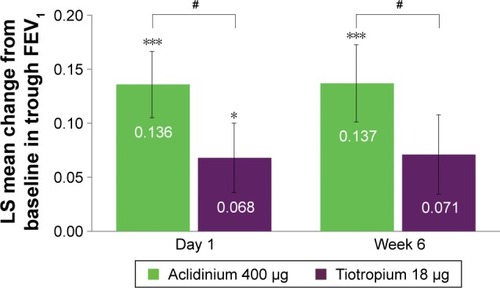
Figure 4 Symptomatic patients: change from baseline in E-RS Total and domain symptom scores versus placebo (A) and percentage of E-RS responders (B) at week 6.
Abbreviations: E-RS, Evaluating Respiratory Symptoms; LS, least squares; OR, odds ratio.
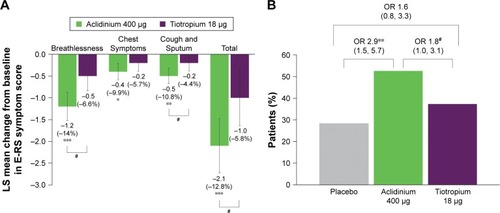
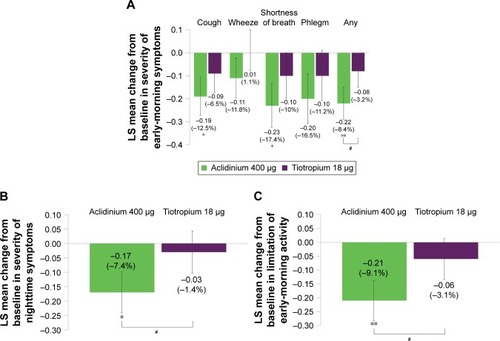
Figure 5 Symptomatic patients: change from baseline in symptom severity in the early morning (A) and nighttime (B), and limitation of early-morning activity (C) over 6 weeks.
Abbreviation: LS, least squares.