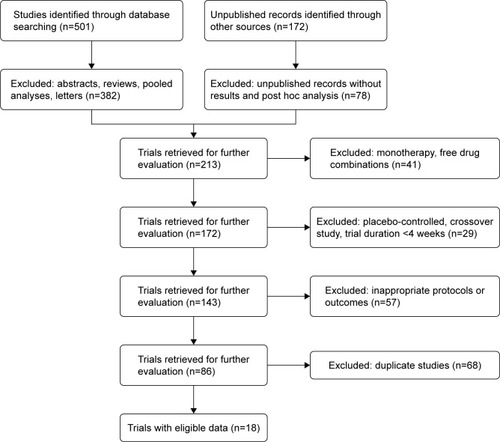
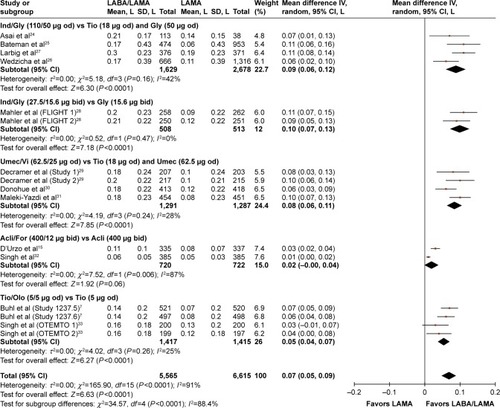
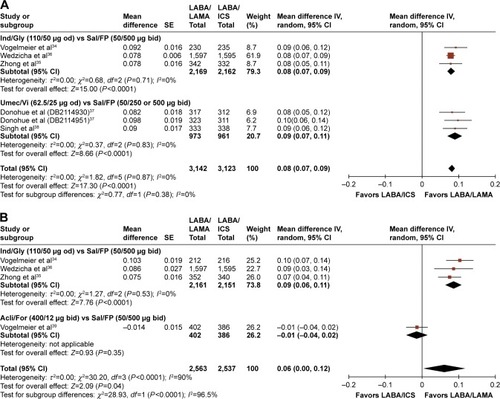
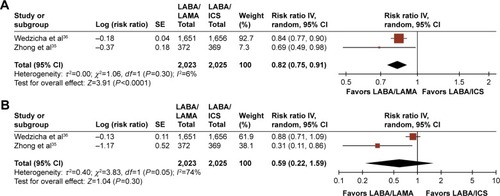
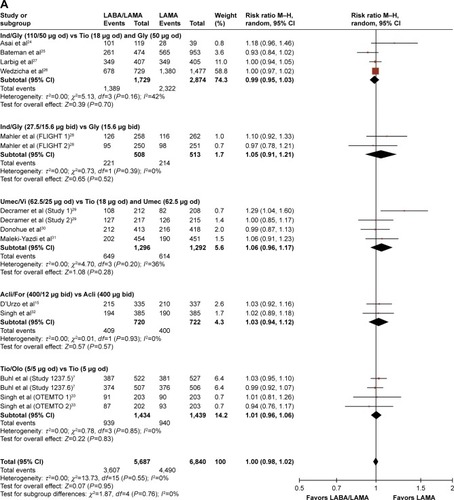
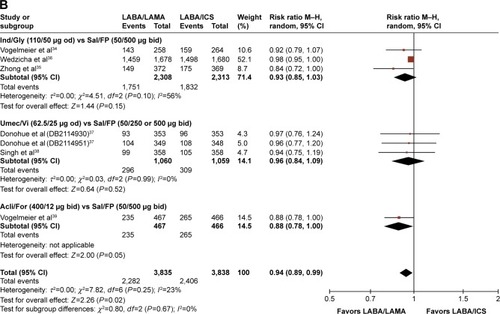
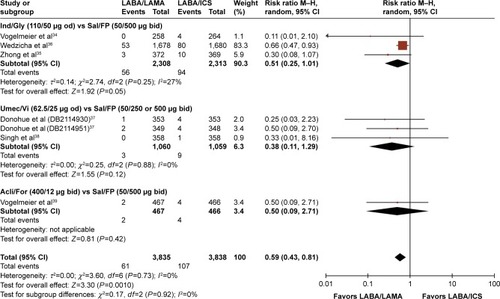
Figures & data
Table 1 Characteristics of included studies
Table 2 Effect of LABA/LAMA versus LAMA or LABA/ICS on trough and peak FEV1
Table 3 Effect of LABA/LAMA versus LAMA or LABA/ICS on secondary COPD outcomes
Table 4 Effect of LABA/LAMA versus LAMA or LABA/ICS on safety outcomes