Figures & data
Table 1 Patient demographic and baseline COPD characteristics (full analysis population)
Figure 1 Patient disposition.
Abbreviations: TIO, tiotropium bromide; FP, fluticasone propionate; FORM, formoterol fumarate.
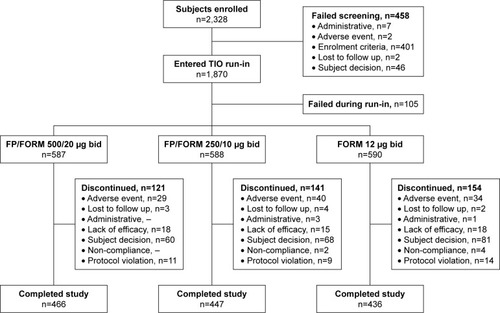
Figure 2 Time to discontinuation.
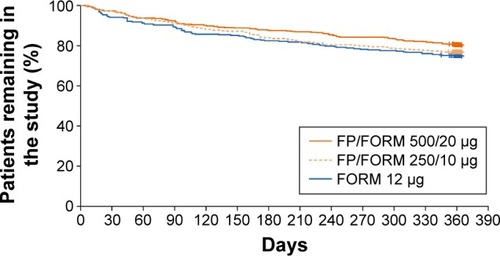
Table 2 Moderate/severe exacerbations analysis (full analysis population)
Table 3 Average pre- and post-dose FEV1 and FVC over the study (full analysis population)
Table 4 EXACT exacerbations analysis (full analysis population)
Figure 3 Change from baseline SGRQ-C.
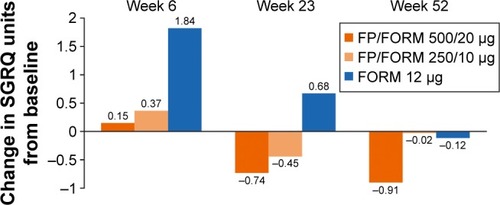
Figure 4 Time to clinically important deterioration.
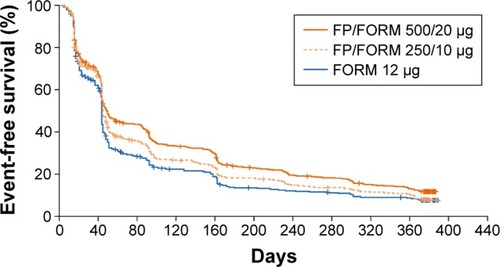
Table 5 Overall incidence of adverse events (safety population)
Table 6 Incidence (%) of frequent adverse events by system organ class (safety population)
