Figures & data
Figure 1 PRISMA flow diagram for the identification of studies included in the meta-analysis concerning the impact of LABA/LAMA FDCs on cardiovascular SAEs in COPD patients.
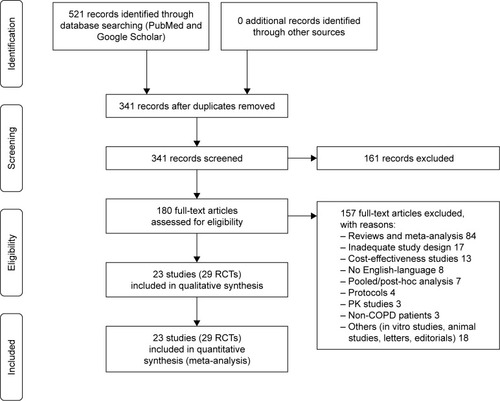
Table 1 Patient demographics, baseline and study characteristics
Figure 2 Forest plot of pair-wise meta-analysis of the impact of the LABA/LAMA FDCs on cardiovascular SAEs in COPD patients.
Abbreviations: A, aclidinium; COPD, chronic obstructive pulmonary disease; F, formoterol; FDCs, fixed-dose combinations; G, glycopyrronium; I, indacaterol; LABAs, long-acting β2-agonists; LAMAs, long-acting muscarinic antagonists; O, olodaterol; SAEs, serious adverse events; T, tiotropium; U, umeclidinium; V, vilanterol.
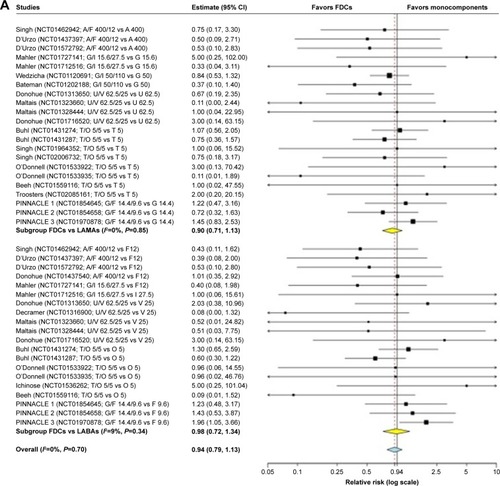
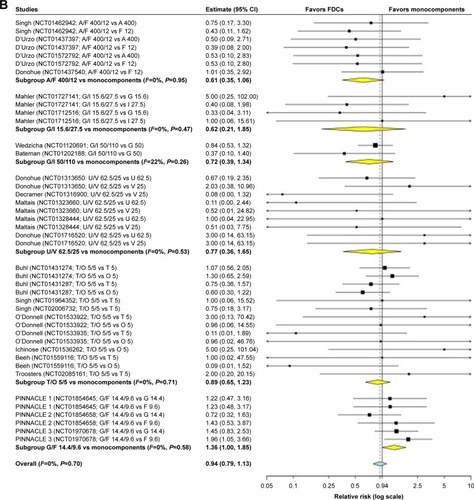
Table 2 Pooled analysis of cardiovascular SAEs extracted from the ClinicalTrials.gov repository database and grouped by frequency in agreement with the EMA guidelineCitation81
Table 3 Safety profile of LABA/LAMA FDCs according to SUCRA analysis
Figure 3 Ranking plot of the network on the cardiovascular safety profile of LABA/LAMA FDCs versus placebo in COPD patients.
Abbreviations: A, aclidinium; COPD, chronic obstructive pulmonary disease; F, formoterol; FDC, fixed-dose combination; G, glycopyrronium; I, indacaterol; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonists; O, olodaterol; PCB, placebo; SUCRA, surface under the cumulative ranking curve; T, tiotropium; U, umeclidinium; V, vilanterol.
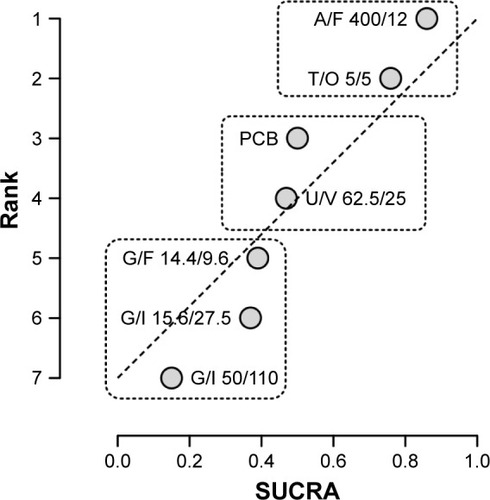
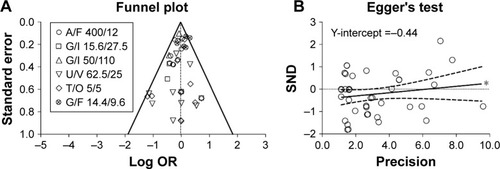
Figure 4 Publication bias assessment via funnel plot (A) and Egger’s test (B) for the impact of LABA/LAMA FDCs on cardiovascular SAEs in COPD patients, versus respective monocomponents.
Abbreviations: A, aclidinium; COPD, chronic obstructive pulmonary disease; F, formoterol; FDCs, fixed-dose combinations; G, glycopyrronium; I, indacaterol; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonists; O, olodaterol; OR, odds ratio; SAEs, serious adverse events; SND, standard normal deviate; T, tiotropium; U, umeclidinium; V, vilanterol.