Figures & data
Table 1 Patient demographics and baseline characteristics (ITT population)
Figure 1 Patient disposition.
Abbreviations: FF, formoterol fumarate; GFF, GP/FF; GP, glycopyrrolate; MDI, metered dose inhaler.
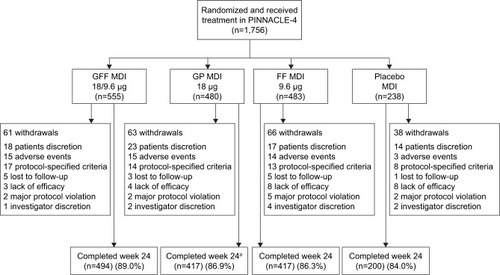
Table 2 Primary and secondary lung function endpoints (ITT population)
Figure 2 LSM change (±SE) from baseline in morning predose trough FEV1 over 24 weeks (ITT population).
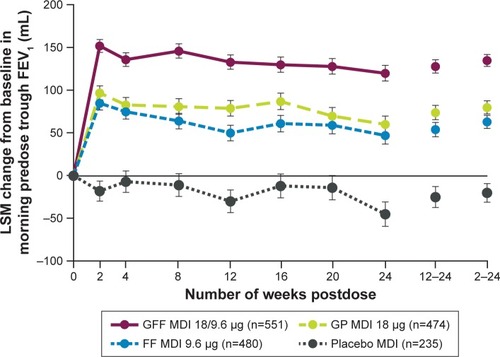
Table 3 Secondary patient-reported outcome endpoints (ITT population, unless stated otherwise)
Table 4 Responder analyses for MCID of secondary, patient-reported outcome endpoints (ITT population)
Table 5 Summary of AEs (safety population)
Table S1 Institutional review boards and approval numbers
Table S2 Additional patient-reported outcome endpoints (ITT population, unless stated otherwise)
