Figures & data
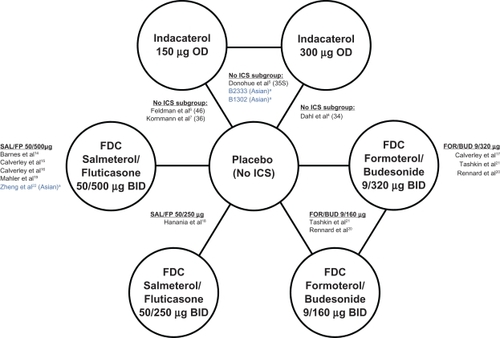
Table 1 Study characteristics for each study included in the network meta-analysis
Table 2 Key baseline patient characteristics for each study included in network meta-analysis
Table 3 Reported data in individual studies included in the network meta-analysis
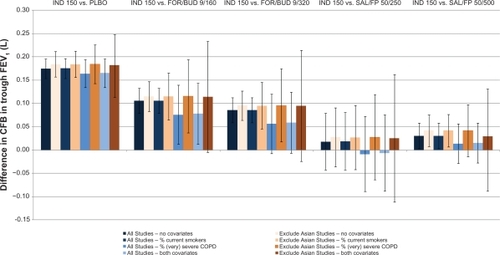
Table 4 Results of network meta-analysis: all treatments versus placebo without covariates
Table 5 Results of network meta-analysis; Indacaterol 150 μg versus alternatives without covariates
Table 6 Results of network meta-analysis: indacaterol 300 μg versus alternatives without covariates
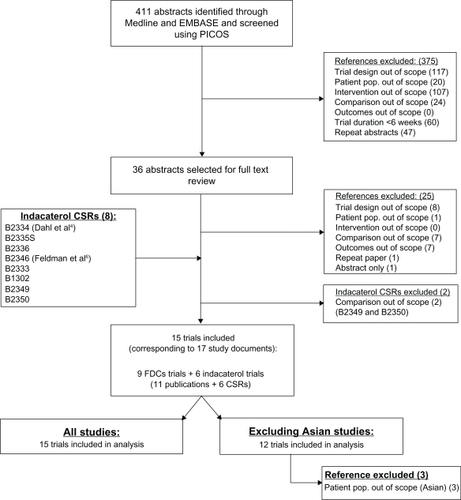
The search strategy was applied for the time period from 1989 to 2009 and 2009 to 2010