Figures & data
Figure 1 Study design.
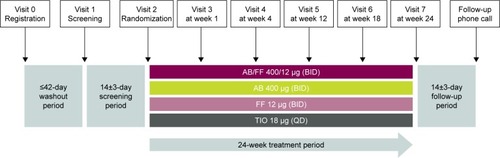
Table 1 Baseline demographics and clinical characteristics (ITT population)
Figure 2 CONSORT flowchart.
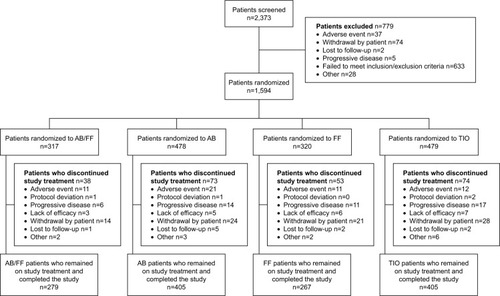
Figure 3 Change from baseline in 1-hour post-dose FEV1 at week 24, ITT population (co-primary endpoint).
Abbreviations: AB, aclidinium bromide; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium.
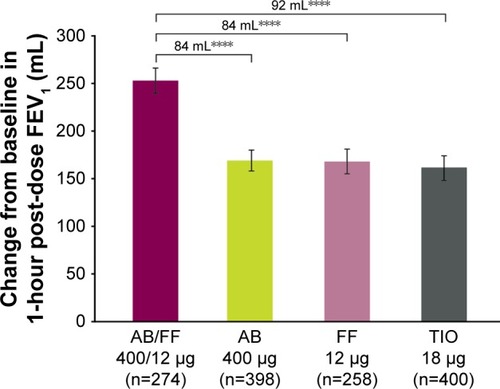
Figure 4 Change from baseline in trough FEV1 at week 24, ITT population (co-primary endpoint).
Abbreviations: AB, aclidinium bromide; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium.
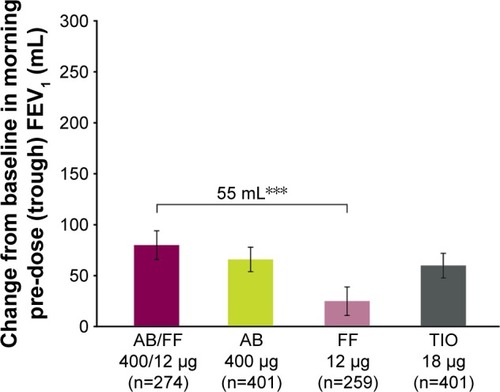
Figure 5 Change from baseline in FEV1 over 3 hours post-dose and AUC0–3/3 h on (A) day 1 and (B) at week 24, ITT population.
Abbreviations: AB, aclidinium bromide; AUC, area under the curve; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium.
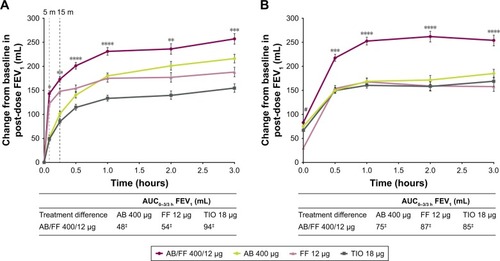
Figure 6 Severity of (A) NiSCI and (B) EMSCI over 24 weeks, ITT population.
Abbreviations: AB, aclidinium bromide; EMSCI, Early-Morning Symptoms of COPD Instrument; FF, formoterol fumarate; ITT, intent-to-treat; NiSCI, Nighttime Symptoms of COPD Instrument; TIO, tiotropium.
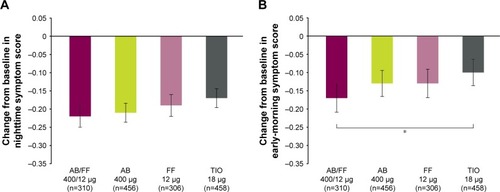
Figure 7 Change from baseline in FEV1 over 24 hours post-dose (A) on day 1 and (B) at week 24, sub-study ITT population.
Notes: *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 for AB/FF vs all other treatments. #P<0.05; ##P<0.01; ###P<0.001; ####P<0.0001 for AB/FF vs FF and TIO. ‡‡‡‡P<0.0001 for AB/FF vs AB and TIO. §P<0.05; §§P<0.01; §§§P<0.001 for AB/FF vs FF. Data are least squares means ± standard error.
Abbreviations: AB, aclidinium bromide; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium bromide.
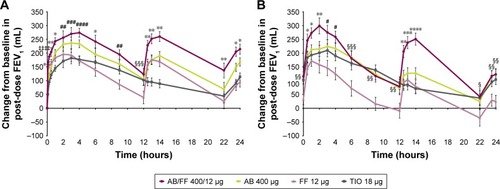
Figure 8 Change from baseline in normalized (A) AUC0–12/12 h, (B) AUC12–24/12 h, and (C) AUC0–24/24 h FEV1 at week 24, sub-study ITT population.
Abbreviations: AB, aclidinium bromide; AUC, area under the curve; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium bromide.
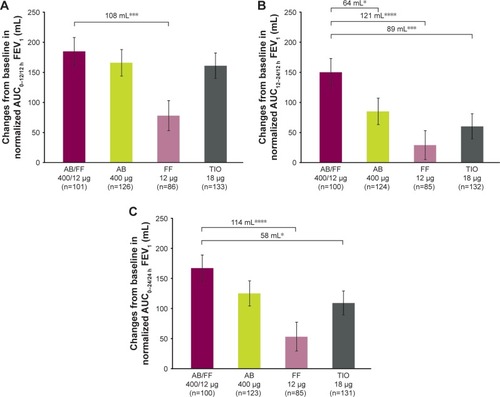
Figure 9 Severity of (A) NiSCI and (B) EMSCI over 24 weeks, sub-study ITT population.
Abbreviations: AB, aclidinium bromide; EMSCI, Early Morning Symptoms of COPD Instrument; FF, formoterol fumarate; ITT, intent-to-treat; NiSCI, Nighttime Symptoms of COPD Instrument; TIO, tiotropium bromide.
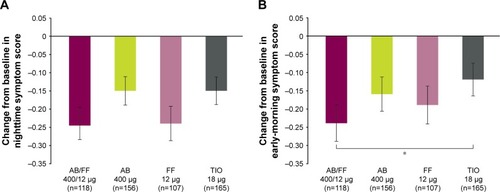
Table 2 Incidence of treatment-emergent adverse events (safety population)
Independent Ethics Committees/Institutional Review Boards and Approval Numbers
Figure S1 Change from baseline in morning pre-dose (trough) FVC (A) at week 1, and (B) week 24, ITT population.
Notes: ***P<0.001; ****P<0.0001. Data are least squares means ± standard error.
Abbreviations: AB, aclidinium bromide; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium bromide.
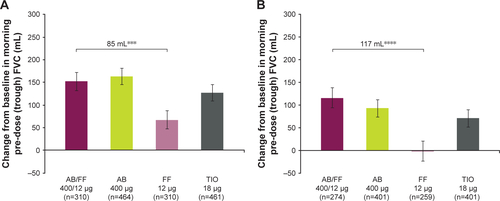
Figure S2 Change from baseline in normalized AUC0–3/3 h FVC (A) on day 1 and (B) at week 24, ITT population.
Notes: **P<0.01; ****P<0.0001. Data are least squares means ± standard error.
Abbreviations: AB, aclidinium bromide; AUC, area under the curve; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium bromide.
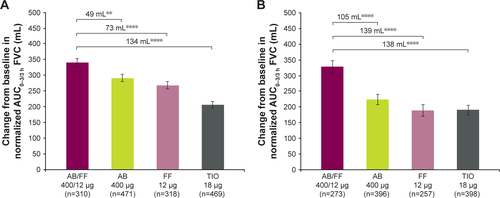
Figure S3 Change from baseline in normalized (A and B) AUC0–12/12 h, (C and D) AUC12–24/12 h, and (E and F) AUC0–24/24 h FVC at day 1 and week 24, sub-study ITT population. Notes: *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. Data are least squares means ± standard error.
Abbreviations: AB, aclidinium bromide; AUC, area under the curve; FF, formoterol fumarate; ITT, intent-to-treat; TIO, tiotropium bromide.
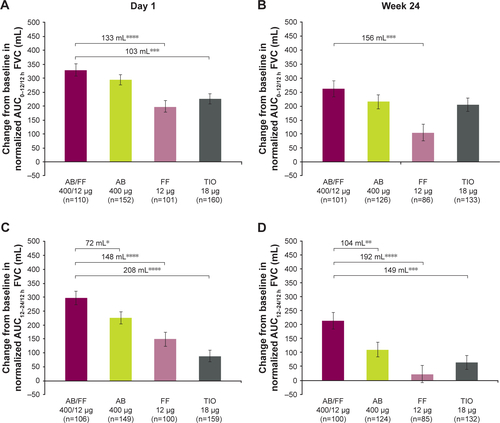
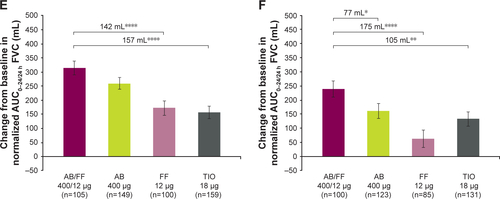
Table S1 Baseline demographics and clinical characteristics (sub-study ITT population)
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
The protocol and the statistical analysis plan can be found at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/View?id=22775.
