Figures & data
Table 1 Studies Investigating The Efficacy Of Maintenance Treatments In Treatment-Naïve Patients With COPD
Figure 1 Treatment differences in trough FEV1 for (A) monotherapies and (B) dual therapies among the relevant publications identified in the literature search.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second.
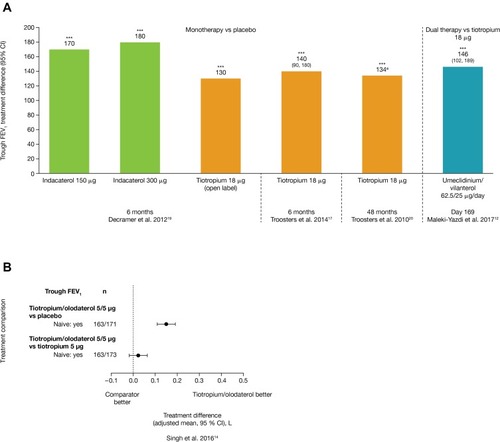
Figure 2 Changes in SGRQ total score for (A) monotherapies and (B) dual therapies; (C) changes in TDI focal score for dual therapies, among the relevant publications identified in the literature search.
Abbreviations: CI, confidence interval; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
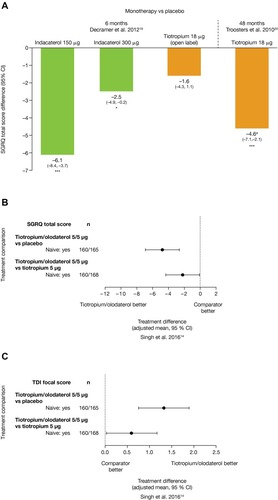
Table 2 Patient Demographics And Baseline Characteristics Of Patients Included In ACLIFORM And AUGMENT (Post-Hoc Analysis; ITT Population)
Figure 3 Change from baseline in (A) 1 hr morning post-dose FEV1 and (B) trough FEV1 for treatment-naïve patients, at Week 24 of ACLIFORM and AUGMENT (post-hoc analysis; ITT population).
Abbreviations: AB, aclidinium bromide; CI, confidence interval; FEV1, forced expiratory volume in 1 sec; FF, formoterol fumarate; ITT, intent-to-treat; LS, least squares.
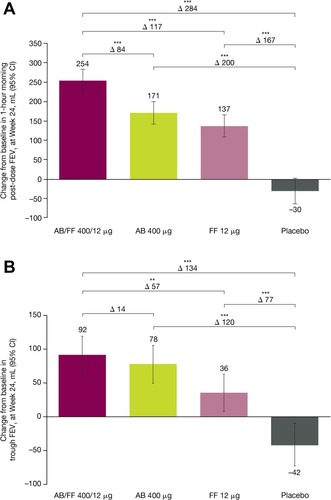
Figure 4 Patient-reported outcomes changes from baseline for treatment-naïve patients (A) TDI focal score at Week 24, (B) E-RS total score, (C) early morning COPD symptom severity and (D) nighttime COPD symptom severity over 24 weeks of ACLIFORM and AUGMENT (post-hoc analysis; ITT population).
Abbreviations: AB, aclidinium bromide; CI, confidence interval; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating-Respiratory Symptoms; FF, formoterol fumarate; LS, least squares; TDI, Transition Dyspnea Index.
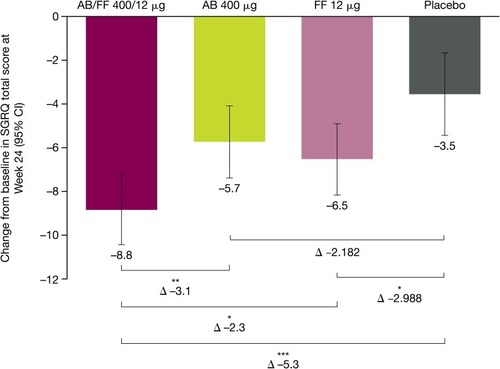
Figure 5 Changes from baseline for SGRQ total score for treatment-naïve patients, at Week 24 of ACLIFORM and AUGMENT (post-hoc analysis; ITT population).
Abbreviations: AB, aclidinium bromide; CI, confidence interval; FF, formoterol fumarate; ITT, intent to treat; SGRQ, St George’s Respiratory Questionnaire.
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
