Figures & data

Figure 1 WISDOM trial design.
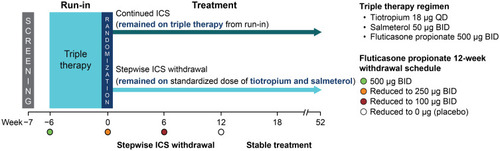
Table 1 Disease and Patient Characteristics at Screening (Patients Taking Triple Therapy at Screeninga and the Overall Trial Population)
Table 2 Time to First COPD Exacerbation and Number of COPD Exacerbations (in Patients Taking Triple Therapy at Screeninga and in the Overall Trial Population)
Figure 2 Kaplan–Meier estimates of the probability of no moderate or severe on-treatment COPD exacerbation in (A) patients taking triple therapy at screening and (B) overall trial population.
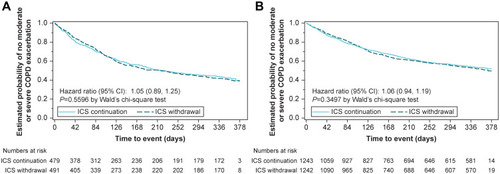
Figure 3 Kaplan–Meier estimates of the probability of no severe on-treatment COPD exacerbation in (A) patients taking triple therapy at screening and (B) overall trial population.
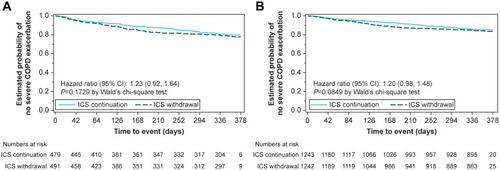
Figure 4 Forest plot of time to first moderate or severe COPD exacerbation in (A) patients taking triple therapy at screening and (B) overall trial population.
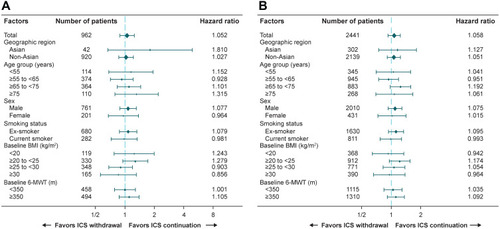
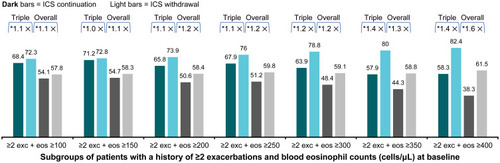
Figure 5 Percentage of patients with at least one moderate or severe COPD exacerbation (in patients taking triple therapy at screeninga and in the overall trial population).
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; eos, blood eosinophil counts; exc, exacerbations; ICS, inhaled corticosteroids.