Figures & data
Figure 1 GOLDEN 3 and GOLDEN 4 study designs: 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter studies.Citation36
Abbreviations: BID, twice daily; CS, closed system; GLY, nebulized glycopyrrolate; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; min, minimum; SAE, serious adverse event; tx, treatment.
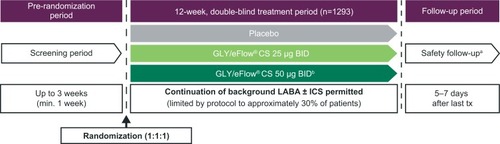
Table 1 Patient Demographics and Baseline Characteristics, by Gender
Figure 2 Pooled analysis of trough FEV1 at 12 weeks, by gender (ITT population).
Abbreviations: BID, twice daily; FEV1, forced expiratory volume in 1 second; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; LS, least squares; SE, standard error.
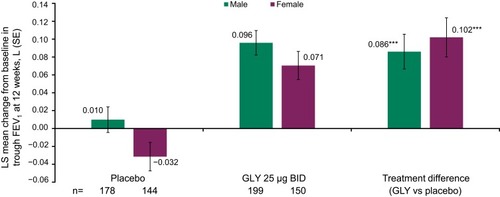
Figure 3 Pooled analysis of (A) SGRQ total score, (B) EXACT-RS total score at 12 weeks, by gender (ITT population).
Abbreviations: BID, twice daily; EXACT-RS, EXAcerbations of COPD Tool-Respiratory Symptoms; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; LS, least squares; SE, standard error; SGRQ, St George’s Respiratory Questionnaire.
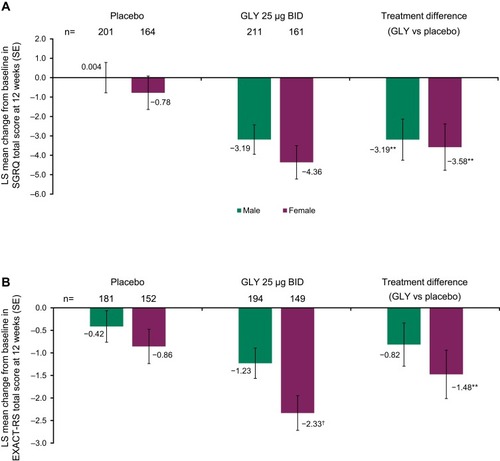
Figure 4 Pooled analysis of (A) SGRQ responder rates, (B) EXACT-RS responder rates at 12 weeks, by gender (ITT population).
Abbreviations: BID, twice daily; CI, confidence interval; EXACT-RS, EXAcerbations of COPD Tool-Respiratory Symptoms; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; OR, odds ratio; SGRQ, St George’s Respiratory Questionnaire.
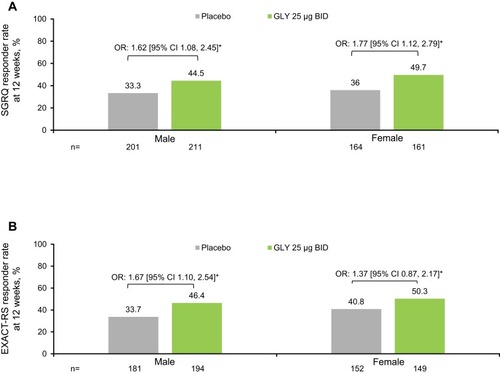
Figure 5 Pooled analysis of rescue medication use over 12 weeks, by gender (ITT population).
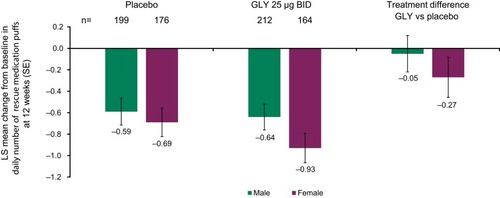
Table 2 Summary of AEs and SAEs, Including Individual AEs with Incidence ≥3% in Any Treatment Group, by Gender (Safety Population)
