Figures & data
Figure 1 GOLDEN 3 and GOLDEN 4 study designs: 12-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter studies.Citation7
Abbreviations: BID, twice daily; CS, closed system; GLY, nebulized glycopyrrolate; GOLDEN, Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist; min, minimum; SAE, serious adverse event; tx, treatment.
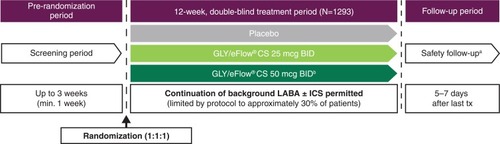
Table 1 Patient Grouping Based on Baseline Rescue Medication Use Quartiles
Table 2 Patient Demographics and Baseline Characteristics by Baseline Rescue Medication Subgroups
Figure 2 Pooled analysis of change from baseline in SGRQ (A) total score, (B) activity, (C) impacts, (D) symptoms component scores, and (E) responders at 12 weeks, by baseline rescue medication use (ITT population).
Abbreviations: BID, twice daily; CI, confidence interval; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; LS, least squares; OR, odds ratio; Q, quarter; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire.
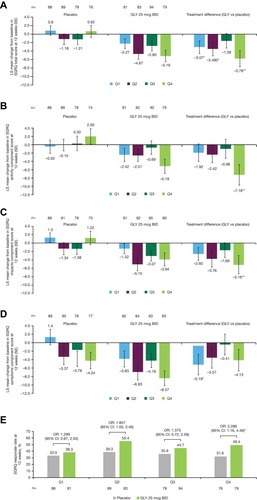
Figure 3 Pooled analysis of change from baseline in EXACT-RS (A) total score and (B) responders at 12 weeks, by baseline rescue medication use (ITT population).
Abbreviations: BID, twice daily; CI, confidence interval; EXACT-RS, EXAcerbations of COPD Tool-Respiratory Symptoms; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; LS, least squares; OR, odds ratio; Q, quarter; SE, standard error.
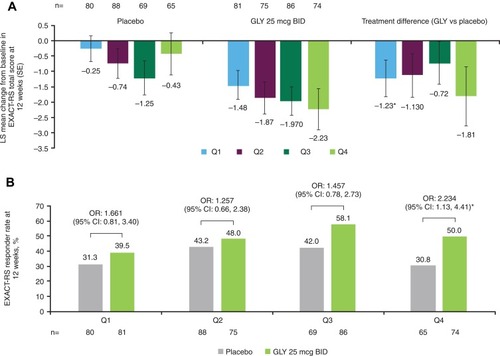
Figure 4 Pooled analysis of change from baseline in trough FEV1 at 12 weeks by baseline rescue medication use subgroup (ITT population).
Abbreviations: BID, twice daily; FEV1, forced expiratory volume in 1 second; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; LS, least squares; Q, quarter; SE, standard error.
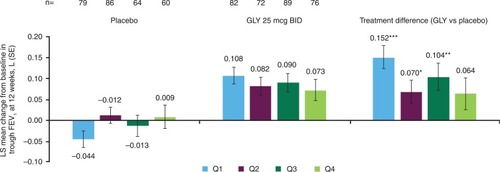
Figure 5 Pooled analysis of change from baseline in the number of puffs of rescue medication per day over 12 weeks by baseline rescue medication use subgroup (ITT population).
Abbreviations: BID, twice daily; GLY, nebulized glycopyrrolate; ITT, intent-to-treat; LS, least squares; Q, quarter; SE, standard error.
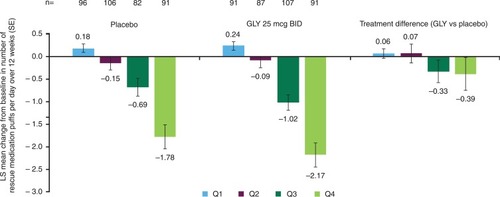
Table 3 Summary of AEs and SAEs, Including Individual AEs with Incidence ≥3% in Any Treatment Group, by Baseline Rescue Medication Use Subgroup (Safety Population)
