Figures & data
Table 1 Patients' Demographic and Anamnestic Data (AB/FF, n=2121; GLY/IND, n=1056; UME/VL, n=476)
Figure 1 LABA/LAMA fixed-dose therapy at baseline (n=3653).
Abbreviations: LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists.
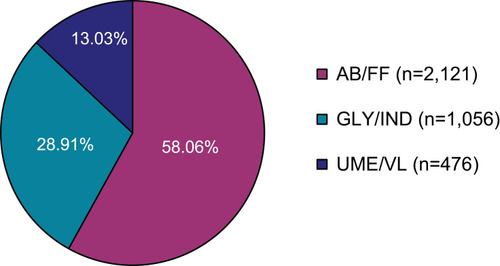
Figure 2 Premature termination of treatment or study (AB/FF, n=2136; GLY/IND, n=1079; UME/VL, n=495). (A) Number of patients who discontinued therapy or study. (B) Most frequent reasons for termination.
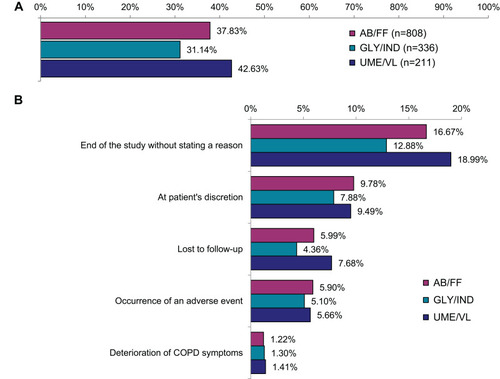
Table 2 Frequency and Severity of Exacerbations During the Study (Patients with Follow-Up Visits; AB/FF, n=1969; GLY/IND, n=1000; UME/VL, n=439)
Figure 3 Lung function during the observational time (LOFC values). (A) Mean FEV1. (B) Mean FVC.
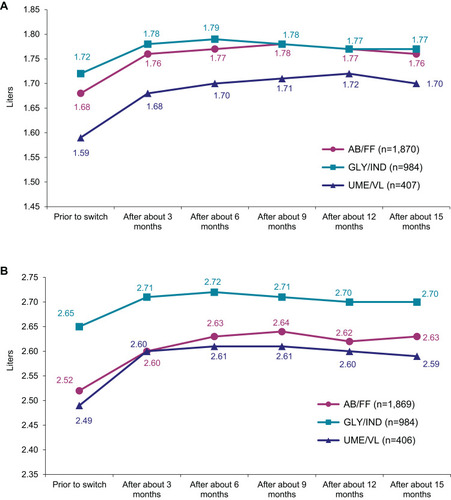
Figure 4 Well-being and quality of life. Mean CAT™ score during the study (LOCF).
Abbreviations: CAT™, COPD Assessment Test; LOCF, Last Observation Carried Forward Method.
Figure 5 Overall severity of early morning COPD symptoms during the course of the study (LOCF).
Abbreviation: LOCF, Last Observation Carried Forward Method.
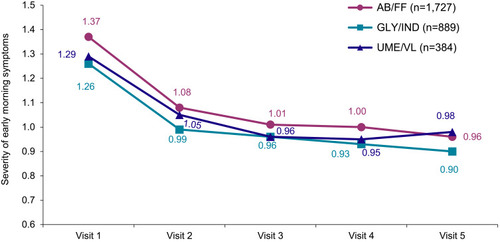
Table 3 Category of Adverse Events and Frequency of Patients Affected (AB/FF, n=2136; GLY/IND, n=1079; UME/VL, n=495)
