Figures & data

Figure 1 Flow chart of patients in the analysis by treatment group, severity of COPD, exacerbation status, and severity of exacerbations (ITT population). All numbers refer to numbers of patients.
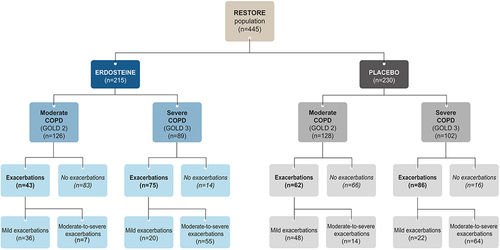
Table 1 Demographic and Baseline Characteristics of Patients (ITT Population)
Figure 2 Mean SGRQ total score for GOLD 2 patients with moderate COPD who experienced exacerbations in each treatment group (erdosteine or placebo) and for the subgroups by exacerbation severity (mild or moderate-to-severe). A lower score represents a better HRQoL. Patients may have experienced more than one exacerbation, but those in the mild exacerbations subgroup only experienced mild exacerbations, while those in the moderate-to-severe exacerbations subgroup may also have experienced mild exacerbations. The n value for each treatment group is the number of patients with exacerbations. There were 127 exacerbations overall (89 mild exacerbations and 38 moderate-to-severe exacerbations). Analysis was conducted in the ITT population and based on ANCOVA model including fixed effects of treatment. P values given above the columns are for significant changes in trend over time for each treatment and for the treatment comparison; they were analyzed using the Residual Maximum Likelihood or least squares method. *P < 0.05 versus placebo at each timepoint.
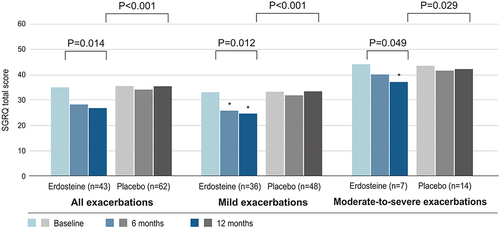
Table 2 Patient Subjective Assessment of Disease Severity Over Time by COPD Severity, Exacerbation Severity and Treatment Group
Figure 3 Proportion of patients with moderate-to-severe exacerbations who used antibiotics alone and with oral corticosteroids by disease severity (GOLD 2 or 3) and treatment group. The percentage value in italics above each stacked bar is the total percentage of patients treated with antibiotics (with or without oral corticosteroids); the remaining patients with moderate-to-severe exacerbations received oral corticosteroids alone. The P values above the columns are for the comparisons of erdosteine versus placebo for the total percentage of patients treated with antibiotics. The asterisks between columns represent *P < 0.05 for erdosteine versus placebo groups within each antibiotic treatment group (antibiotic + oral corticosteroid or antibiotic alone). Analysis used a Chi-square test followed by Fisher’s exact test.
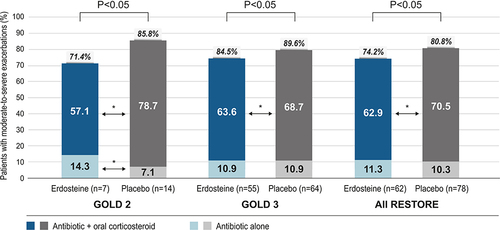
Table 3 Oral Corticosteroid Total Dose and Average Daily Dose (Prednisolone-Equivalents) Over 12 Months in Patients Experiencing Moderate-to-Severe Exacerbations by COPD Severity and Treatment Group
