Figures & data
Table 1 Baseline demographics, background characteristics, and spirometry (safety population)
Table 2 Differences between treatments for primary and secondary efficacy outcomes on day 1 and at week 12 (FAS)
Figure 3 Trough FEV1 after first dose (end of day 1) and week 12 (FAS).
Abbreviations: FAS, full analysis set; FEV1, forced expiratory volume in 1 second.
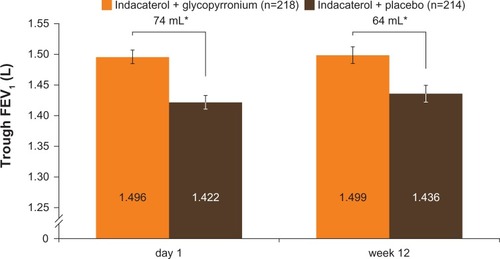
Figure 4 FEV1 from 30 minutes to 4 hours postdose and 24 hours postdose (A) on day 1 and (B) at week 12 (FAS).
Abbreviations: FEV1, forced expiratory volume in 1 second; FAS, full analysis set.
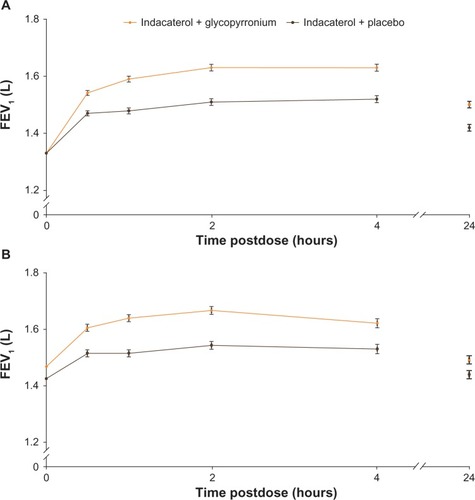
Figure 5 Subgroup analyses of treatment differences in trough FEV1 at week 12 (FAS).
Abbreviations: BMI, body mass index; CI, confidence interval; FAS, full analysis set; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; LSM, least squares mean; N1, number of patients analyzed in the indacaterol + glycopyrronium treatment group; N2, number of patients analyzed in the indacaterol + placebo treatment group.
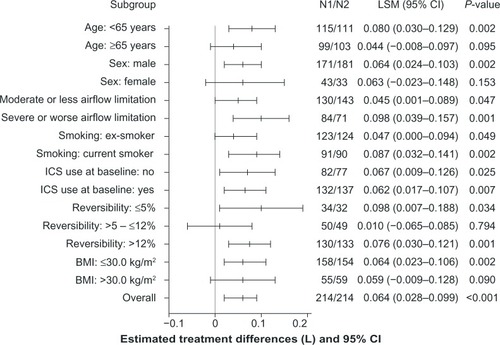
Figure 6 TDI focal score at week 12 (FAS).
Abbreviations: FAS, full analysis set; TDI, transition dyspnea index.
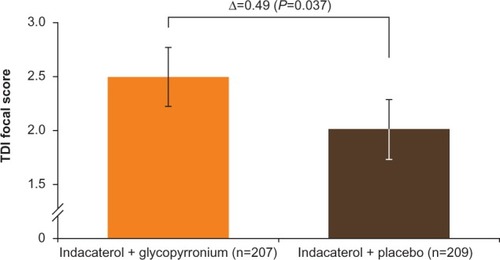
Table 3 Most frequent AEs (at least three patients in either treatment group) and discontinuations due to AEs (safety population), n (%)
Table 4 SAEs (safety population), n (%)
Table S1 List of study centers
Table S2 Medications allowed in the GLOW6 study under certain conditions
Table S3 Procedure for handling missing data in the GLOW6 study