Figures & data
Figure 1 Patient selection.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; OPCRD, Optimum Patient Care Research Database; QOF, quality and outcomes framework.
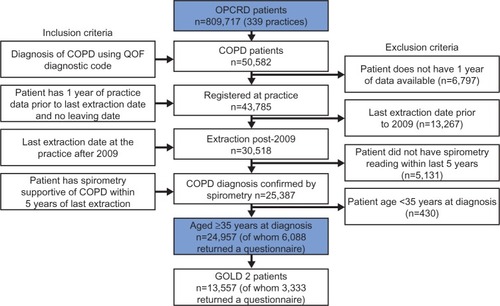
Table 1 Patient demographics for the total COPD population split by GOLD group
Table 2 Patient demographics for the GOLD Stage 2 population split by GOLD group
Figure 2 Distribution of GOLD groups in patients without and with moderate and severe exacerbations in the year prior to data extraction for total COPD population (A) and GOLD Stage 2 subset (B).
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
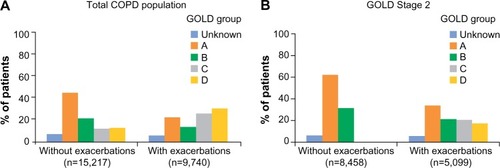
Figure 3 Current management for total COPD population (A) and GOLD Stage 2 subset (B) by GOLD groups.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; SAMA, short-acting muscarinic antagonist; SABA, short-acting β2-agonist.
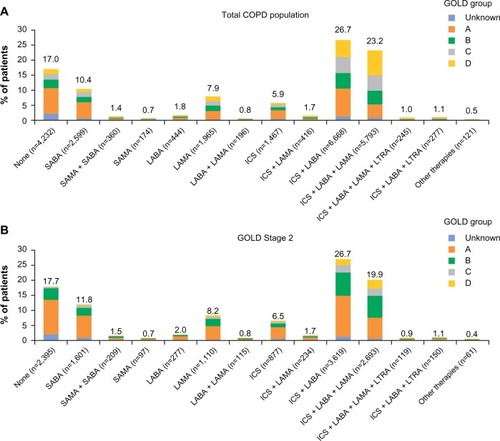
Figure 4 Current management by concomitant asthma diagnosis for total COPD population (A) and GOLD Stage 2 subset (B).
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; SAMA, short-acting muscarinic antagonist; SABA, short-acting β2-agonist.
Figure 5 Current management by moderate and severe exacerbation rate in the year prior to data extraction for the GOLD Stage 2 subset.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; SAMA, short-acting muscarinic antagonist; SABA, short-acting β2-agonist.
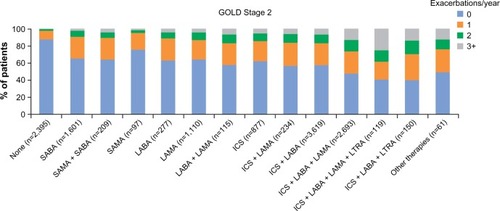
Figure 6 Current management by mMRC score (A) and CAT score (B) for the GOLD Stage 2 subset.
Abbreviations: CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council; LTRA, leukotriene receptor antagonist; SAMA, short-acting muscarinic antagonist; SABA, short-acting β2-agonist.
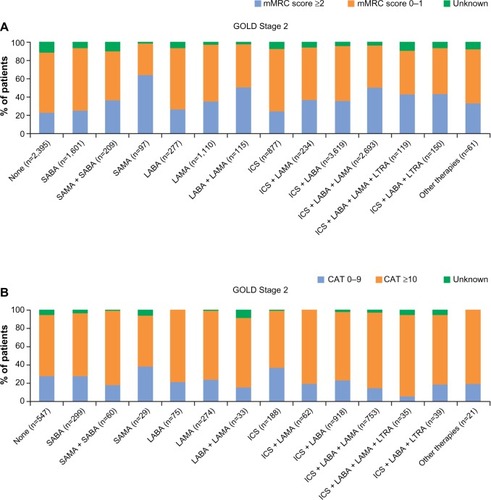
Figure S1 Model of symptom/risk evaluation of COPD.
Notes: When assessing risk, choose the highest risk according to GOLD stage or exacerbation history (one or more hospitalizations for COPD exacerbations should be considered high risk). GOLD 1, 2, 3, and 4 correspond to mild (FEV1 ≥80% predicted), moderate (50% ≤ FEV1 <80% predicted), severe (30% ≤ FEV1 <50% predicted), and very severe (FEV1 <30% predicted) airflow limitation, respectively. Reproduced from the Global Strategy for Diagnosis, Management and Prevention of COPD 2014. Copyright © Global Initiative for Chronic Obstructive Lung Disease (GOLD), all rights reserved. Available from http://www.goldcopd.org.Citation1
Abbreviations: CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council.
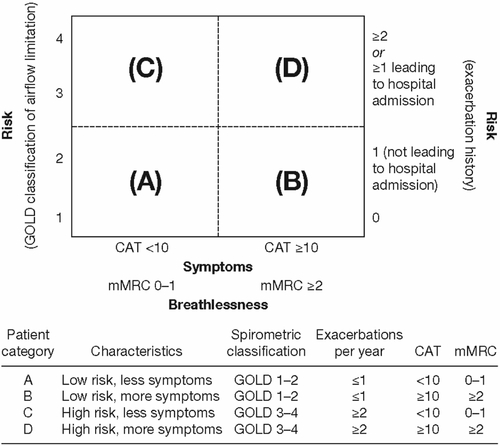
Figure S2 Modified Medical Research Council questionnaire for assessing the severity of breathlessness.
Reproduced from the Global Strategy for Diagnosis, Management and Prevention of COPD 2014. Copyright © Global Initiative for Chronic Obstructive Lung Disease (GOLD), all rights reserved. Available from http://www.goldcopd.org.Citation1
Abbreviation: mMRC, modified Medical Research Council.
Figure S3 COPD Assessment Test.
Notes: The COPD Assessment Test score is calculated as the sum of the responses present. If more than two responses are missing, a score cannot be calculated; when one or two items are missing their scores can be set to the average of the nonmissing item scores. Reproduced with permission. COPD Assessment Test and CAT logo is a trade mark of the GlaxoSmithKline group of companies. © 2009 GlaxoSmithKline group of companies. All rights reserved.Citation2
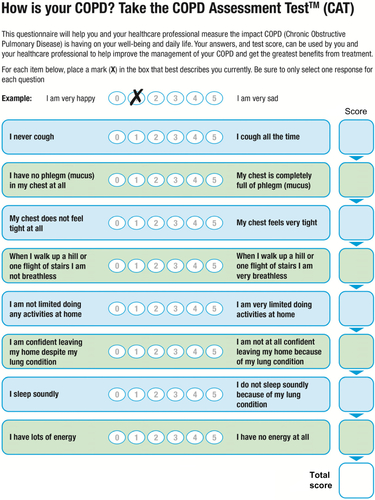
Table S1 Characteristics of patients with COPD who were responders or nonresponders to the study questionnaires
