Figures & data
Table 1 Overview of Phase II, Phase III, and long-term trials of aclidinium in COPD
Figure 1 Study designs for the two pivotal Phase III studies of aclidinium BID: (A) ACCORD COPD I,Citation31 and (B) ATTAIN.Citation32
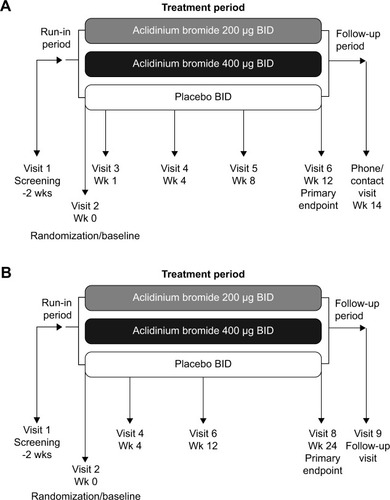
Figure 2 Change from baseline in (A) trough FEV1 and (B) peak FEV1 at Week 24 in ACCORD COPD I study.
Notes: *P<0.001 vs placebo; †P<0.05, ‡P<0.01 vs aclidinium 200 μg. From Kerwin EM, D’Urzo AD, Gelb AF, et al. COPD 2012;9(2):90–101. Copyright © 2012, Informa Healthcare. Reproduced with permission of Informa Healthcare.Citation31
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; FEV1, forced expiratory volume in 1 second; LS, least squares; SE, standard error.
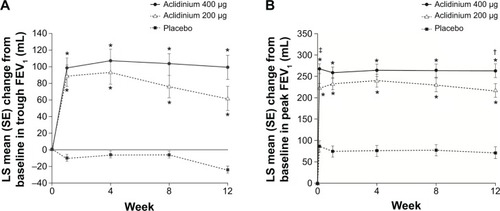
Figure 3 Change from baseline in (A) trough FEV1 and (B) peak FEV1 at Week 24 in ATTAIN study.
Abbreviations: ATTAIN, Aclidinium To Treat Airway obstruction In COPD patieNts; BID, twice daily; FEV1, forced expiratory volume in 1 second.
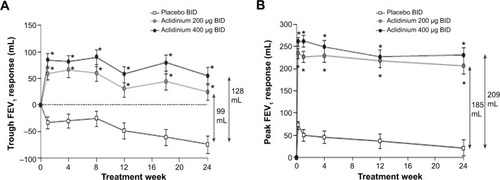
Figure 4 Least squares mean (standard error) change from baseline in SGRQ total score in patients on continuous aclidinium in 1-year extension study of ACCORD COPD I.
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; SGRQ, St George’s Respiratory Questionnaire.
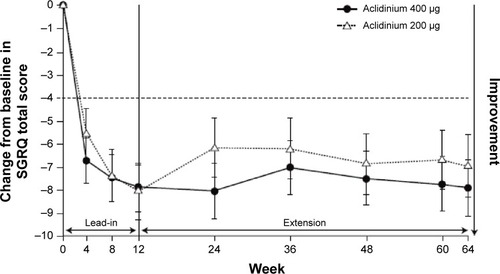
Figure 5 Percent change from baseline in frequency of night-time COPD symptoms at Week 12 in the ACCORD COPD I study.
Note: †P≤0.0023 vs placebo.
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; BID, twice daily.
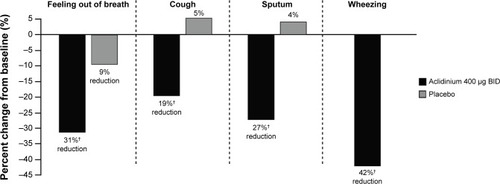
Figure 6 Percent change from baseline in severity and impact of early morning symptoms at Week 12 in the ACCORD COPD I study.
Notes: †P=0.0009, ‡P=0.0002.
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; BID, twice daily.
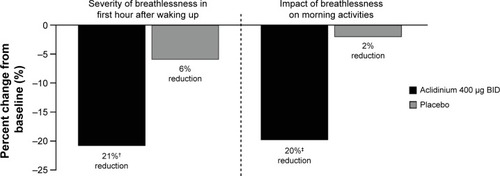
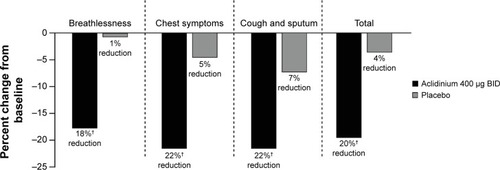
Figure 7 Percent change from baseline in daily COPD symptoms as measured by EXACT-RS scores at Week 24 in the ATTAIN study.
Abbreviations: ATTAIN, Aclidinium to Treat Airway Obstruction in COPD Patients; BID, twice daily; EXACT-RS, EXAcerbations of Chronic pulmonary disease Tool-Respiratory Systems.