Figures & data
Table 1 Baseline demographics and characteristics
Figure 1 Patient disposition.
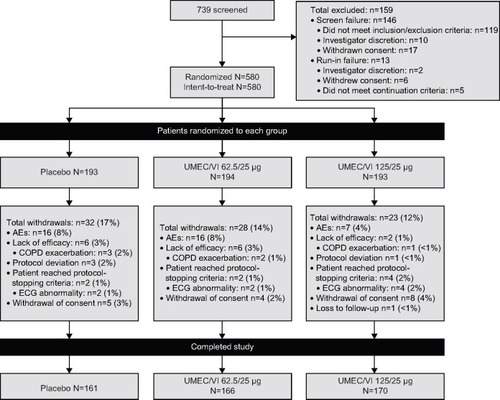
Table 2 Summary of lung-function, symptomatic, and health-related quality of life measures (ITT population)
Figure 2 LS mean change from baseline in trough FEV1 up to day 169 (ITT population).
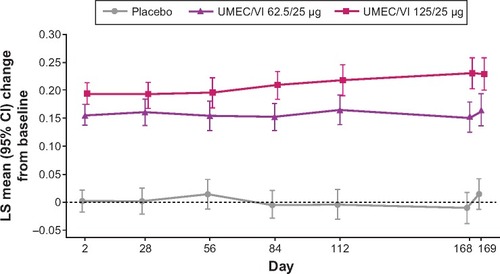
Figure 3 LS mean change from baseline in serial FEV1 on day 1 (ITT population).
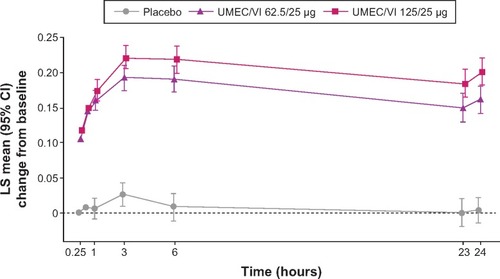
Figure 4 LS mean TDI focal score (ITT population).
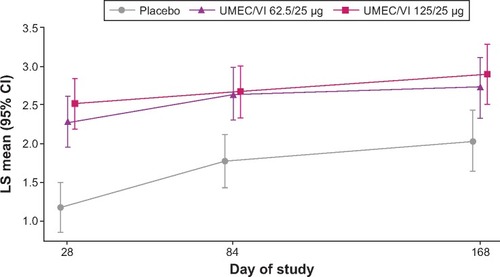
Table 3 Overall summary of AEs (ITT population)
Table S1 Medications prior to screening: use of the medications according to the defined time intervals prior to visit 1
Table S2 Analysis of SGRQ total score (ITT population)
