Figures & data
Figure 1 Flow chart of study selection process.
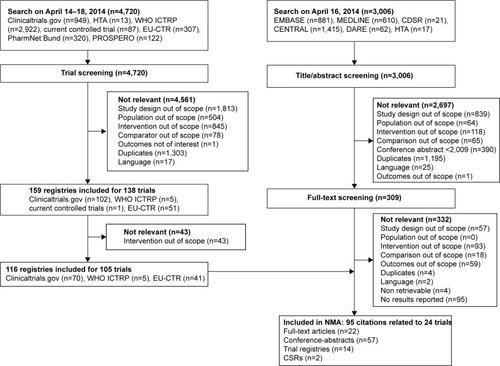
Table 1 Key study characteristics for all studies included (only arms of interest)
Table 2 Key patient characteristics at baseline for all studies included (only arms of interest)
Figure 2 Overall network of studies in the network meta-analysis of umeclidinium versus other LAMAs or placebo for (A) trough FEV1 at 12 weeks, (B) trough FEV1 at 24 weeks, (C) SGRQ total score at 24 weeks, (D) TDI focal score at 24 weeks, and (E) rescue medication use at 24 weeks.
Abbreviations: BD, twice-daily; FEV1, forced expiratory volume in 1 second; LAMA, long-acting muscarinic antagonist; OD, once-daily; PBO, placebo; SGRQ, St George’s Respiratory Questionnaire; TDI, transitional dyspnea index.
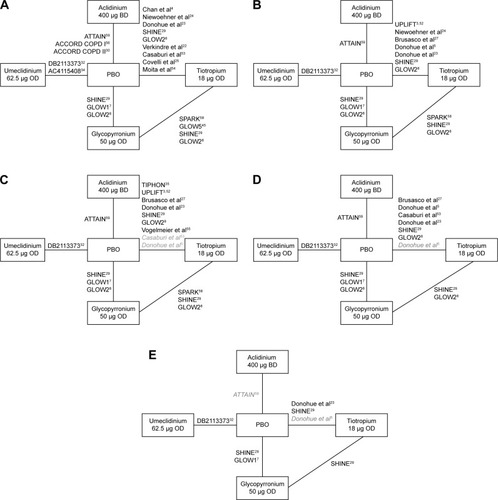
Table 3 Individual study results for trough FEV1, SGRQ total scores, TDI focal scores, and rescue medication use
Figure 3 Differences in intervention versus the comparator for change from baseline in (A) trough FEV1 (mL) at 12 weeks, (B) trough FEV1 (mL) at 24 weeks, (C) SGRQ total scores at 24 weeks, (D) TDI focal scores at 24 weeks, and (E) rescue medication use at 24 weeks (95% CrI and probability of the intervention being better than the comparator).
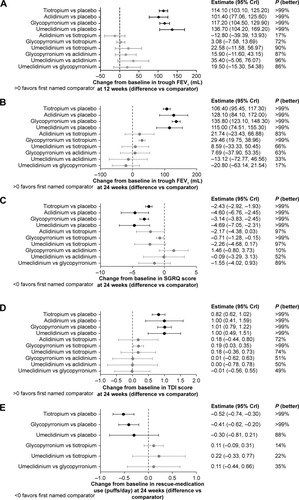
Table S1 Search strategy for the systematic review
Table S2 Participants, interventions, comparisons, outcomes, and study design (PICOS) criteria
Table S3 Data extraction
Table S4 Risk of bias assessment for the included studies
Table S5 Individual study results for trough SGRQ total scores, TDI focal scores, and rescue medication use
Table S6 Differences in intervention versus the comparator for change for SGRQ total scores, TDI focal scores, and rescue medication use at 12 weeks (95% CrI and probability of the intervention being better than the comparator)
