Figures & data
Table 1 The forward primers of miRNAs
Table 2 The primers of target gene mRNAs
Figure 1 Diabetic hCEC identification and the effect of ICA II on cell proliferation and migration in diabetic hCECs.
Abbreviations: hCEC, human cavernous endothelial cell; ICA II, icariside II; eNOS, endothelial nitric oxide synthase; RAGE, receptor for advanced glycation end products; NC, normal control; DAPI, 4′,6-diamidino-2-phenylindole; DM, diabetes mellitus; DM+ICA II, DM treated with ICA II.
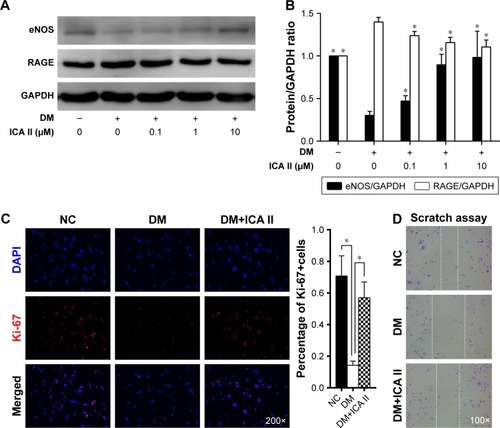
Figure 2 Effect of ICA II on endothelial miRNAs and miR-126/SPRED1 expression in diabetic hCECs.
Abbreviations: ICA II, icariside II; miRNA, microRNA; SPRED1, sprouty-related EVH1 domain-containing protein 1; hCEC, human cavernous endothelial cells; PIK3R2, phosphoinositol-3 kinase regulatory subunit 2; VCAM1, vascular cell adhesion molecule 1; NC, normal control; DM, diabetes mellitus; DM+ICA II, DM treated with ICA II.
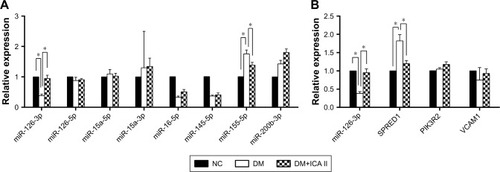
Figure 3 Effects of ICA II on regulating protein expression levels in the MAPK pathway in diabetic hCECs.
Abbreviations: ICA II, icariside II; hCECs, human cavernous endothelial cells; SPRED1, sprouty-related EVH1 domain-containing protein 1; NC, normal control; DM, diabetes mellitus; DM+ICA II, DM treated with ICA II.
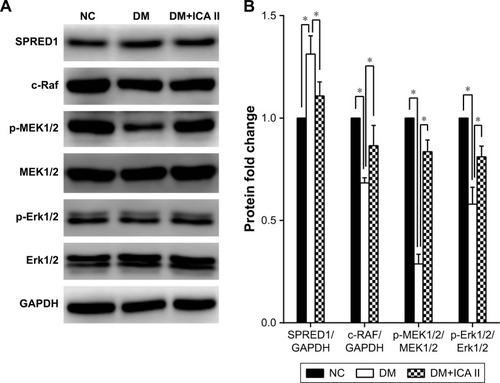
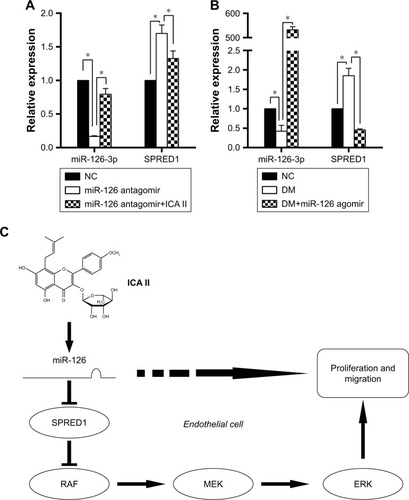
Figure 4 The influence of miR-126-3p on SPRED1 regulation and the hypothetical scheme of ICA II on endothelial cell function in diabetic hCECs.
Abbreviations: SPRED1, sprouty-related EVH1 domain-containing protein 1; ICA II, icariside II; DM, diabetes mellitus; hCECs, human cavernous endothelial cells; NC, normal control.