Figures & data
Figure 1 Structure of the chelating drugs.
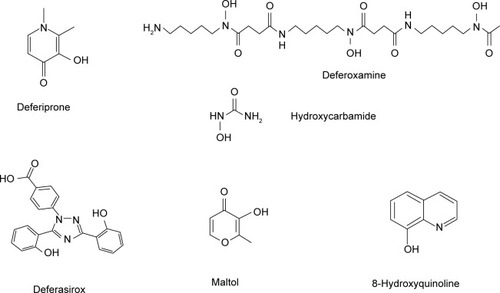
Table 1 Examples of thalassemia intermedia and other conditions where iron overload can be caused from the increased gastrointestinal absorption of ironTable Footnote*
Figure 2 Iron-absorption and iron-overload mechanisms in non-transfusion-dependent thalassemias: the role of chelators and chelating drugs.
Abbreviations: DMT1, divalent metal transporter 1; EDTA, ethylenediaminetetraacetic acid; DTPA, diethylenetriaminepentaacetic acid.
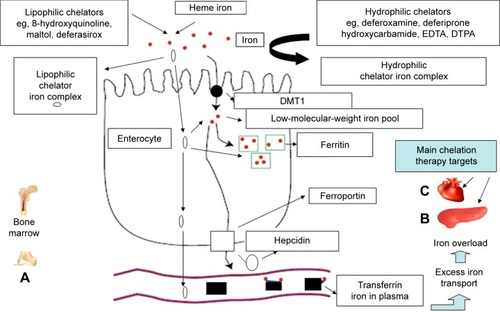
Table 2 Examples of nonregulatory factors affecting iron absorptionTable Footnote*
Table 3 Mode of action and property differences of the chelating drugs deferoxamine, deferiprone, and deferasirox and other chelatorsTable Footnote*
