Figures & data
Table 1 ATR SNEDDS and NS experimental runs showing factor combinations
Figure 1 Ternary phase diagrams of ATR SNEDDS with the composition of various components.
Abbreviations: ATR, atorvastatin; SNEDDS, self-nanoemulsifying drug delivery systems.
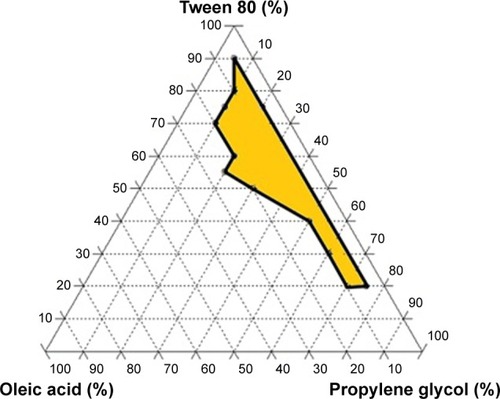
Table 2 Observed responses of all formulations of experimental design
Table 3 Analysis of variance (ANOVA) results of multiple regression analysis of the investigated responses
Table 4 Estimated effects and associated P-values for all three responses
Figure 2 Quantile–quantile plots for predicting the dependent variables.
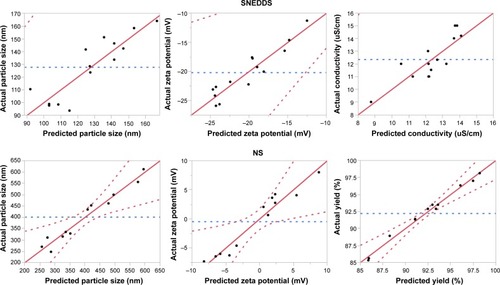
Figure 3 Standard Pareto charts showing the effects of independent variables and their combined effects on the designed responses of SNEDDS and NS formulations.
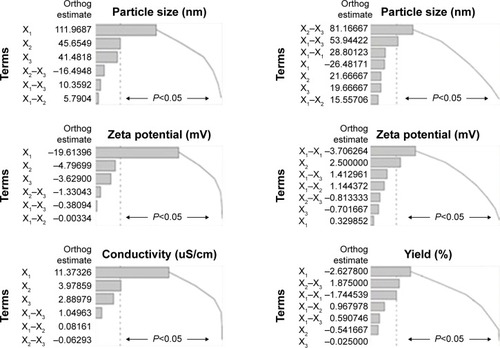
Figure 4 TEM image of optimized SNEDDS formula (A) and SEM image of optimized NS formula (B).
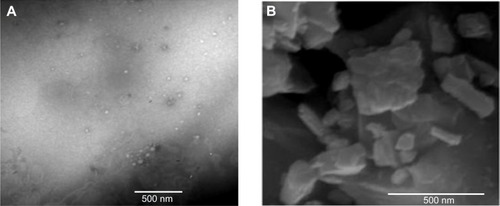
Figure 5 In vitro release of optimized SNEDDS and NS formulations.
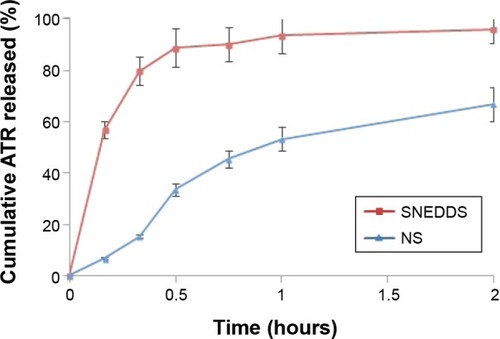
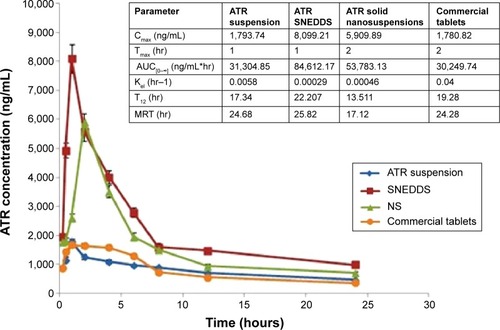
Figure 6 Means of plasma concentrations-time profiles and in vivo pharmacokinetic parameters (inset) of optimized ATR SNEDDS and optimized ATR solid NS versus ATR suspension and commercial tablets.
Abbreviations: ATR, atorvastatin; SNEDDS, self-nanoemulsifying drug delivery systems; NS, nanosuspensions; Cmax, Maximum plasma level; AUC[0–], area under curve; hr, hour; Ke, elimination rate constant; T1/2, half life time; MRT, mean residence time.