Figures & data
Table 1 Sequences of primers of S. mansoni applied in the current study (Durand et al 2000). GenBank accession numbers for these loci are also shown
Table 2 In vitro effects of PSO and PZQ on mortality of adult worms, juveniles and schistosomula of S. mansoni after different incubation periods
Table 3 Activity of adult Schistosoma mansoni in PSO- and PZQ-treated groups after different incubation periods
Figure 1 In vitro mortality rate of adult male and female Schistosoma mansoni of differently treated groups at 72 hours’ exposure.
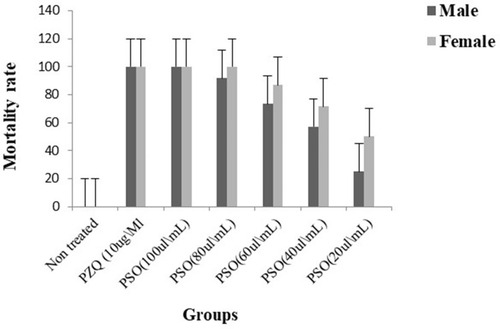
Figure 2 Scanning electron microscopy of Schistosoma mansoni adult male worminteguments. After in vitro incubation for 72 hours in medium with 1% DMSO (negative control group): (A) intact oral suckers (OS), ventral suckers (VS), and well-developed tubercles (T). Bar 200 µm; (B) higher magnification showing well-developed tubercles (T), spines (S), and tegumental ridges (TR) on the dorsal surface (bar 20 µm). PZQ-treated worms after 72 hours’ incubation: (C) anterior ends exhibited deformity of ventral suckers (VS) (bar 10 µm); (D) distorted tubercles (DT) with loss of spines (LS) and areas of surface peeling (bar 10 µm) (E); and severe erosion of tubercles with multiple holes (H), bleb formation (B), and exposed muscle layer (M) in some (bar 50 µm). Worms exposed to PSO for 72 hours at 60 µg/mL concentration: (F) destruction of integument (DT) and loss of normal tubercular pattern, with peeling areas (P) and deformity of ventral suckers (VS) (bar 20 µm); (G) and distortion and flattening of gynecophoric canal (FGC). bar 200 µm). At higher PSO concentration (100 µg/mL): (H) worms showed edematous integuments with more damage (bar 10 µm); (I) deformity of tubercles, loss of spines, and tegumental ridges, with formation of blebs (B) and peeling (P; bar 10 µm); (J) deformity and edematous ventral suckers (EVS) with tegumental peeling (P), laceration, complete loss of spines, and destruction of the integument (bar 10 µm); and (K) erosion, marked laceration, loss of spines (S), and extermination of tubercles (ET) with bleb formation (bar 20 µm).
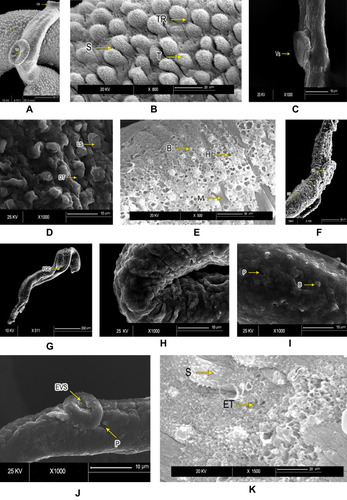
Table 4 Activity of immature juvenile Schistosoma mansoni in PSO- and PZQ-treated groups after different incubation periods (IPs)
Table 5 Activity of Schistosoma mansoni schistosomula in PSO- and PZQ treated groups after different incubation periods (IPs)
Figure 3 Scanning electron micrography of Schistosoma mansoni juvenile wormsafter in vitro incubation for 72 hours in medium with DMSO (negative-control group) showed (A) intact oral (OS) and ventral suckers (VS) and primitive ill-developed gynecephoric canal (PGC), whereas the dorsal surface was covered with rows of tegumental folds (TF; bar 400 µm). At 72 hours after exposure to PSO at 100 µg/mL concentration, worms exhibited (B) areas of laceration (L) and peeling (P), (C) multiple vesicles (V) and blebs (B) (bars 400 µm and 200 µm, respectively), (D) and some showed dorsal bending (DB) of the worm body (bar 400 µm).
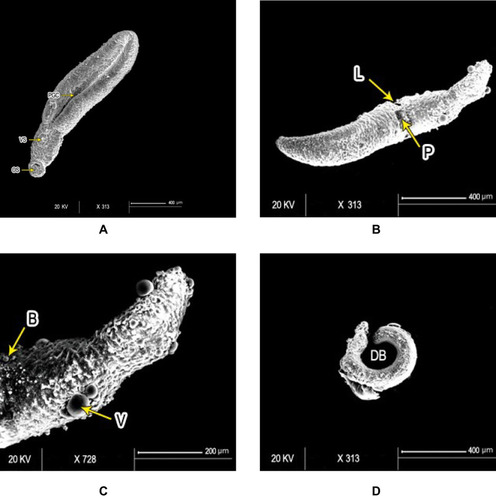
Figure 4 Scanning electron micrography of S. mansoni schistosomula. Ultrastructure of Sschsiistosomula incubated in medium with 1% DMSO (control group) exhibited (A) intact integument with relatively flat surface and slight tegumentary ridges (STR; bar 100 µm) and (B) an apical constriction (AC) between the head and the body region. At the posterior end of the body, there was a prominent tail-socket (TS) wound left by the breakage of the tail (bar 300 µm). After 72 hours of exposure to PSO, Sschsiistosomula showed (C) swelling of body with obvious wrinkling (W) and focal lesions (FL). There was an elongation of the worm, which may indicate flaccid paralysis (bar 200 µm). (D) Blisters (BL), blebs (B), and vesicles scattered on the surface (bar 50 µm) at high concentration (100 µL/mL) of PSO.
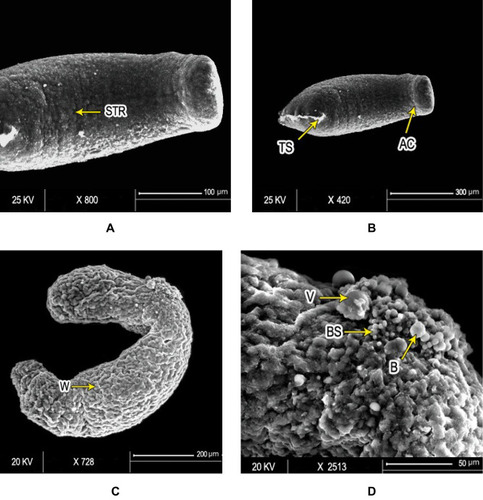
Figure 5 Lanes 2–7 after the 1 kb plus DNA-ladder PCR profiles for S. mansoni microsatellite loci R95529 (A) and SMD57 (B) in adult worms treated with 100 µL/mL PSO (lane 1), 40 µL/mL PSO (lane 2), 20 µL/mL PSO (lane 3), PZQ (Lane 4), schistosomula treated with 100 µL/mL PSO (lane 5), schistosomula sham control (lane 6), and adult schistosomula sham control (lane 7). A negative PCR control was loaded in lane 8 in both gels.
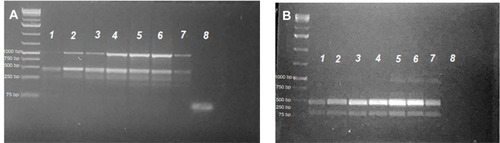
Figure 6 Densitometric measurements of alleles-band intensity for the microsatellite locus R95529 in adult worms treated with 100 µL/mL PSO (100% PSO), 40 µL/mL PSO (40% PSO), 20 µL/mL PSO (20% PSO), praziquantel (PZQ), untreated adult (NT-A) schistosomula treated with 100 µL/mL PSO (S100% PSO), and schistosomula untreated control (NT-S). Letters a–d refer to significant differences (p<0.05) in comparison to the control of each tested maturation (adult schistosomula) group.
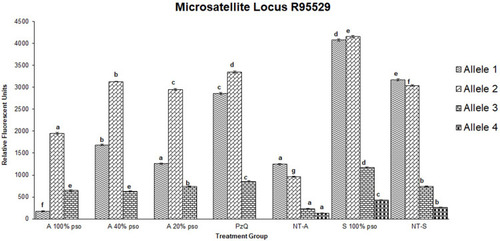
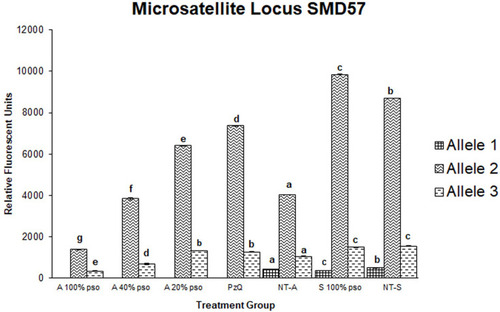
Figure 7 Densitometric measurements of alleles-bands intensities for the microsatellite locus SMD57 in adult worms treated with 100 µL/mL PSO (100% group), 40 µL/mL PSO (40% group), 20 µL/mL PSO (20% PSO), praziquantel (PZQ), untreated adult (NT-A), schistosomula treated with 100 µL/mL (S100% PSO), and schistosomula control (NT-S). Letters a–d refer to significant differences (p<0.05) in comparison to the control of each tested maturation (adult schistosomula) group.