Figures & data
Figure 1 Three pathways of latent infection of HSV. (A) IE gene is adequate, the early expression gene is increased and largely neurons are cleaved and subsequently cleared. Due to the low copy number of HSV DNA in neurons, DNA synthesis is reduced and residual viruses are latent infection. (B) IE gene expression can produce ICP0 protein. ICP0 activates the JNK signaling pathway, and AP-1 is phosphorylated, then activate LAT. LAT sequence is complementary to the IE gene encoding ICP0, which continuously consumes ICP0, so decrease IE gene expression. ICP0 and LAT increase histone occupancy and heterochromatin levels of lytic genes and block their transcription, causing HSV latency. (C) Low levels of ICP0 can promote the expression of LAT and lytic genes in latently infected ganglion, where the loading of total histone and heterochromatin on HSV genome was increased, maintaining latency. LAT transcription can downregulate lytic gene transcription during latency. Moreover, ICP0 promoted AP-1 nuclear translocation, which promotes the expression of LAT. Subsequently many products of LAT (LAT, MiR and ORF) are produced, which will make HSV latent through anti-apoptotic pathway.
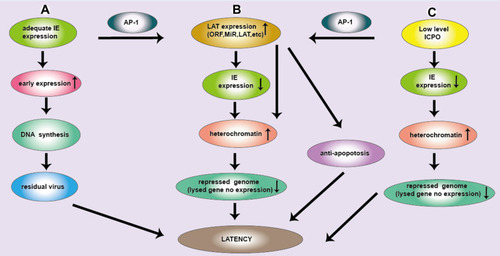
Figure 2 The LAT locus and (twintron) splicing of the HSV genome. (A) HSV-1 includes UL and US regions with terminal and internal repeats (TRL, IRL, IRS and TRS). LAT1 is in TRL and UL, LAT2 is in IRL and UL connection region. An TRL fragment, overlapping ICP0, ICP34.5, and ICP4, is expanded to show LAT1, including 8.3 kb original LAT, and 2 kb and 1.5 kb of the LAT intron. The 8.3 kb LAT2 was spliced into a 2.0 kb intron and a 6.3 kb mRNA or spliced as twintron introns into a 0.5 kb unstable intron and a 7.8 kb RNA. (B) HSV-2, like HSV-1, contains the LAT1 and LAT2. Their transcripts include an unstable 9.0 kb primary LAT and a stable 2.2 kb primary LAT.
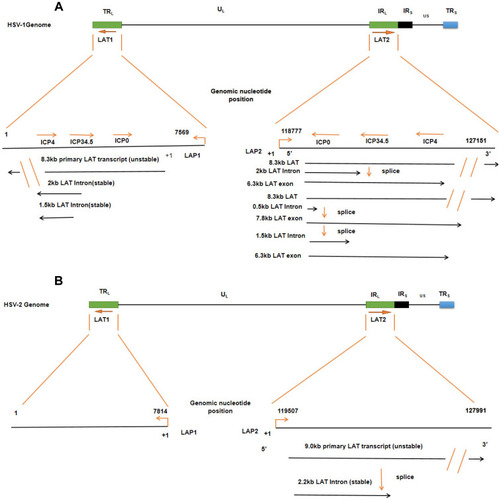
Figure 3 Regulation of LAT expression and function of LAT. (A–C) highlight the pathways that promote the transcriptional expression of LATs. 1–5 shows the five functional mechanisms of LAT latency. 1. LAT miR-H2 can inhibit ICP0 protein so that viral DNA cannot be synthesized, causing the silencing of the lysed gene and HSV latency. LAT miRNA-H6 inhibit the expression of ICP4 and lysed genes. LAT miRNA blocks ICP0/ICP34.5 production and reduces viral DNA synthesis, which in turn inhibits the expression of the lysed gene and renders the virus latent. LAT miRNA can also achieve viral latency by targeting transformed growth factor (TGF)-β and SMAD3 in the TGF-β pathway to resist apoptosis. 2. LAT sRNA1 and sRNA2 can inhibit ICP0 mRNA expression by partial base complementation to maintain latency. LAT sRNA2 can also inhibit the expression of ICP4 and lysed genes to help establish and maintain virus latency. 3. LAT lncRNA can induce facultative heterochromatin to promote lytic gene silencing during latency. 4. LAT sncRNA collaborates with RIG-I to stimulate NF-kB dependent transcription for anti-apoptosis and latency. 5. LAT ORFs inhibit ICP4 and then down-regulates lysed genes to make the virus latent. At the same time, LAT ORFs inhibit caspase 8 or caspase 9 for anti-apoptosis and latency.
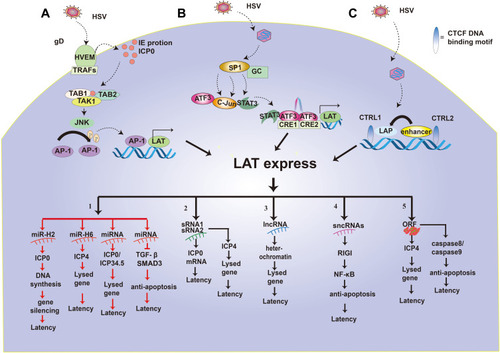
Table 1 Application of Deleted LAT in HSV
Table 2 Application of Deleted LAT in oHSV
