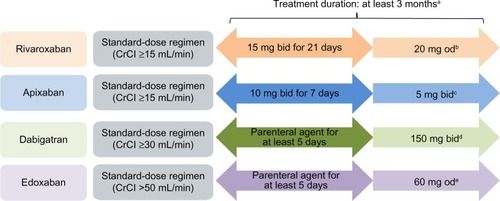
Notes: Rivaroxaban, dabigatran, apixaban, and edoxaban are approved in the EU.
aThe duration of therapy should be individualized after careful assessment of the treatment benefit against the risk of bleeding. Short duration of therapy (at least 3 months) should be based on transient risk factors (eg, recent surgery, trauma, immobilization) and longer durations should be based on permanent risk factors or idiopathic DVT/PE.
bA reduction in the dose from 20 mg od to 15 mg od should be considered if the patient’s assessed risk of bleeding outweighs the risk of recurrent DVT/PE. Rivaroxaban should be used with caution in patients with CrCl 15–29 mL/min and is not recommended in patients with CrCl <15 mL/min.
10 cFor the prevention of recurrent DVT or PE following completion of 6 months of treatment for DVT or PE, 2.5 mg bid is recommended. Apixaban should be used with caution in patients with CrCl 15–29 mL/min and is not recommended in patients with CrCl <15 mL/min.
11 dDabigatran 110 mg bid is recommended in patients aged ≥80 years and those who receive concomitant verapamil. In the following patient groups, the dose (150 mg bid or 110 mg bid) should be selected based on individual assessment of the thromboembolic and bleeding risks: patients aged 75–80 years; patients with moderate renal impairment; those with gastritis, esophagitis, or gastroesophageal reflux; and other patients at increased risk of bleeding. Dabigatran is contraindicated in patients with severe renal impairment (CrCl <30 mL/min).
8 eEdoxaban 30 mg od is recommended in patients with one or more of the following clinical factors: moderate or severe renal impairment (CrCl 15–50 mL/min), low body weight (≤60 kg), or concomitant use of the following P-gp inhibitors: cyclosporin, dronedarone, erythromycin, or ketoconazole.
Citation9Abbreviations: bid, twice daily; CrCl, creatinine clearance; DVT, deep-vein thrombosis; EU, European Union; od, once daily; NOAC, non-vitamin K antagonist oral anticoagulant; PE, pulmonary embolism; P-gp, P-glycoprotein; VTE, venous thromboembolism.