Figures & data
Table S1 Physicochemical properties of SPIONLA-HSA and SPIONLA-HSA-Ptx particles
Table S2 Impact of free Ptx and SPIONLA-HSA-Ptx on breast cancer cell lines
Figure 1 Physicochemical properties of Ptx-loaded SPIONs.
Notes: (A) Hydrodynamic diameter of Ptx-loaded and unloaded particles in water and different cell culture media. (B) Dialysis-based release kinetics of SPION-adsorbed Ptx compared with free Ptx. (C, D) ζ potential as a function of pH in SPIONLA-HSA and SPIONLA-HSA-Ptx during (C) forward titration and (D) backward titration. (E) Magnetization curves showing the M(H) data of SPIONLA-HSA and SPIONLA-HSA-Ptx. (F) AC susceptibility spectra of SPIONLA-HSA and SPIONLA-HSA-Ptx. (G, H) Fourier transform infrared spectroscopy spectra of (G) SPIONLA-HSA, SPIONLA-HSA-Ptx, and (H) free Ptx. (I) Stability of SPIONLA-HSA and SPIONLA-HSA-Ptx in human blood. SPIONs coated with lauric acid and aminated human serum albumin (SPIONLA-HSA-NH2) were used as a positive control for nonstable particles. Negative control = corresponding amount of H2O instead of water-based ferrofluid. Representative images were recorded using optical bright-field microscopy.
Abbreviations: DMEM, Dulbecco’s Modified Eagle’s Medium; EDTA, ethylenediaminetetraacetic acid; FBS, fetal bovine serum; H, applied magnetic field; M φ, magnetization; Ptx, paclitaxel; RPMI, Roswell Park Memorial Institute; SPION, superparamagnetic iron oxide nanoparticles; SPIONLA-HSA, lauric acid- and human serum albumin-coated SPIONs; SPIONLA-HSA-NH2, lauric acid- and aminated human serum albumin-coated SPIONs; SPIONLA-HSA-Ptx, SPIONLA-HSA functionalized with Ptx; Xi, real part of the magnetic susceptibility; Xii, imaginary part of the magnetic susceptibility.
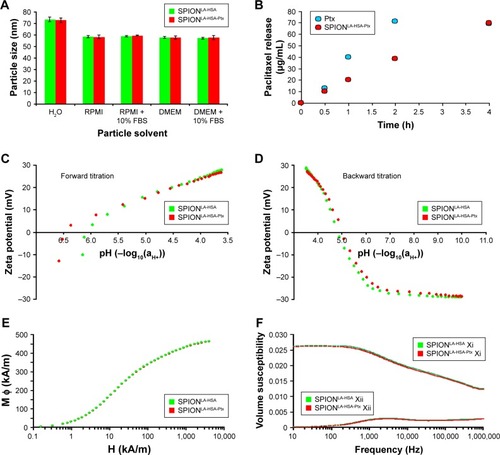
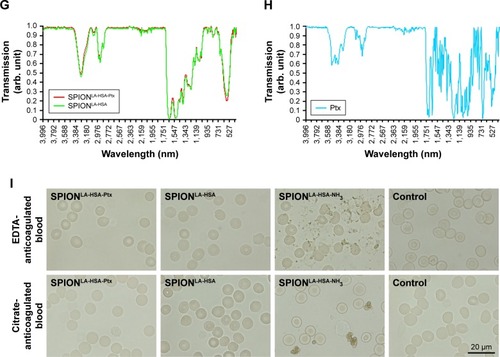
Figure 2 Viability of breast cancer cells 48 hours after Ptx treatment.
Notes: (A) BT-474, (B) MCF-7, (C) MDA-MB-231, and (D) T-47D cells were incubated for 48 hours with increasing amounts of free Ptx, SPIONLA-HSA-Ptx, and SPIONLA-HSA, and analyzed by multiparameter flow cytometry. Viability was determined by AxV–FITC and PI staining (first column), yielding the percentage of viable (Ax− PI−), apoptotic (Ax+ PI−), and necrotic (PI+) cells. The status of the mitochondrial membrane potential was analyzed by DiIC1(5) staining and distinguishes cells with intact (DiIC1(5) positive) and depolarized (DiIC1(5) negative) membranes (middle column). DNA degradation and cell cycle were determined by PIT staining and showed the amount of degraded DNA, diploid DNA (G1 phase), and double-diploid DNA (synthesis/G2 phase; last column). Positive controls contain 2% DMSO, and negative controls represent the corresponding amount of solvent instead of drug or ferrofluid. Data are expressed as the mean ± SD (n=3 with technical triplicate). Statistical significance of viability, intact membrane potential, and diploid DNA content between control and samples are indicated with *P<0.01, **P<0.001, and ***P<0.0001, and were calculated via Student’s t-test analysis.
Abbreviations: AxV, Annexin A5; DiIC1(5), 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide; DMSO, dimethyl sulfoxide; FITC, fluorescein isothiocyanate; PI, propidium iodide; PIT, propidium iodide–Triton X-100; Ptx, paclitaxel; SPION, superparamagnetic iron oxide nanoparticles; SPIONLA-HSA, lauric acid- and human serum albumin-coated SPIONs; SPIONLA-HSA-Ptx, SPIONLA-HSA functionalized with Ptx.
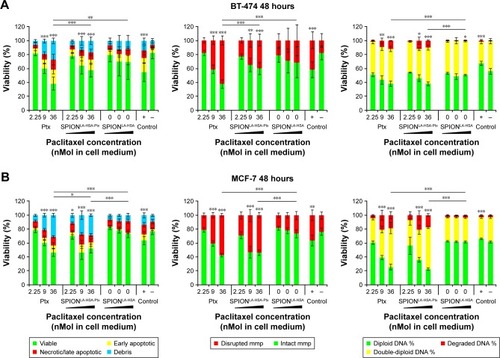
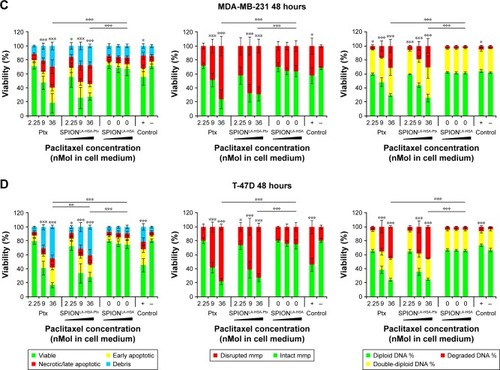
Figure 3 Growth kinetics of different cell lines in the presence of Ptx and SPIONLA-HSA-Ptx.
Notes: (A) BT-474, (B) MCF-7, (C) MDA-MB-231, and (D) T-47D cells were incubated for 7 days with increasing amounts of free Ptx, SPIONLA-HSA-Ptx, and SPIONLA-HSA. Cellular confluency was determined hourly using the IncuCyte® live-cell imaging system, providing growth kinetics for all different samples. Positive controls contained 2% DMSO, and negative controls contained the corresponding amount of solvent instead of drug or ferrofluid. Data are expressed as the mean ± SD (n=4 with technical triplicate).
Abbreviations: Ctrl, control; DMSO, dimethyl sulfoxide; Ptx, paclitaxel; SPION, superparamagnetic iron oxide nanoparticles; SPIONLA-HSA, lauric acid- and human serum albumin-coated SPIONs; SPIONLA-HSA-Ptx, SPIONLA-HSA functionalized with Ptx.
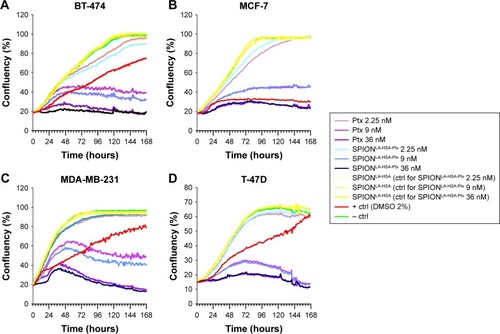
Figure 4 Effects of SPIONLA-HSA-Ptx and Ptx on three-dimensional cell clusters.
Notes: (A, B) BT-474, (C, D) MCF-7, (E, F) MDA-MB-231, and (G, H) T-47D spheroids were treated with 36 nM free Ptx or particle-bound Ptx (SPIONLA-HSA-Ptx). In addition, a SPIONLA-HSA carrier control, a SPION- and Ptx-free negative control, and positive toxicity control containing 2% DMSO were utilized. The first column displays the projected two-dimensional area during the treatment. The second column shows representative pictures of the spheroids during the first 144 hours after treatment.
Abbreviations: DMSO, dimethyl sulfoxide; Ptx, paclitaxel; SPION, superparamagnetic iron oxide nanoparticles; SPIONLA-HSA, lauric acid- and human serum albumin-coated SPIONs; SPIONLA-HSA-Ptx, SPIONLA-HSA functionalized with Ptx.
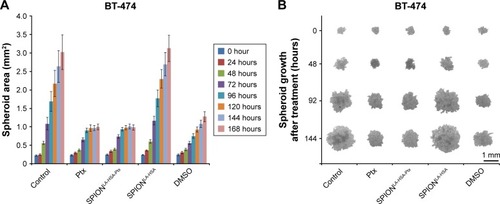
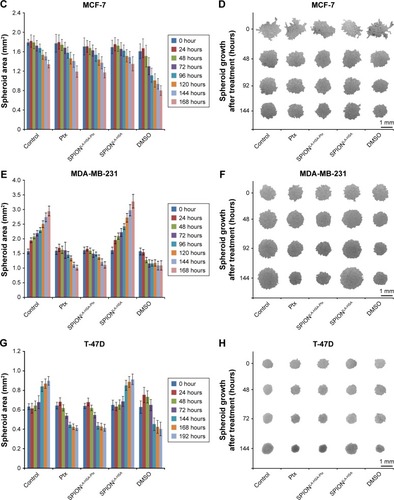
Figure S1 Effect of different solvents on the size of SPIONLA-HSA-Ptx and SPIONLA-HSA.
Notes: SPIONLA-HSA-Ptx and SPIONLA-HSA were diluted in (A, B) H2O, (C, D) RPMI, (E, F) RPMI + 10% FBS, (G, H) DMEM, and (I, J) DMEM + 10% FBS. Z-average and PDI were analyzed on day 1 after preparation and after 7 days of storage at 4°C by DLS, and the results are depicted in representative graphs. The strong increase in the PDI in samples containing 10% FBS is obviously the result of the additional FBS signal at 7.3 nm.
Abbreviations: DLS, dynamic light scattering; DMEM, Dulbecco’s Modified Eagle’s Medium; PDI, polydispersity index; Ptx, paclitaxel; SPION, superparamagnetic iron oxide nanoparticles; SPIONLA-HSA, lauric acid- and human serum albumin-coated SPIONs; SPIONLA-HSA-Ptx, SPIONLA-HSA functionalized with paclitaxel; RPMI, Roswell Park Memorial Institute; Z-average, intensity-weighted harmonic mean size.
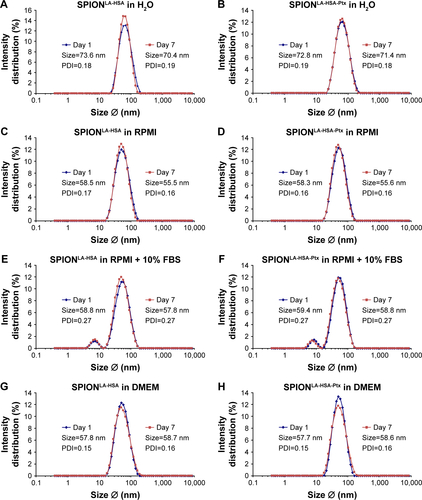
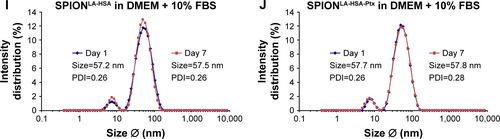
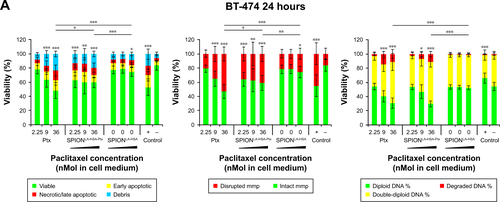
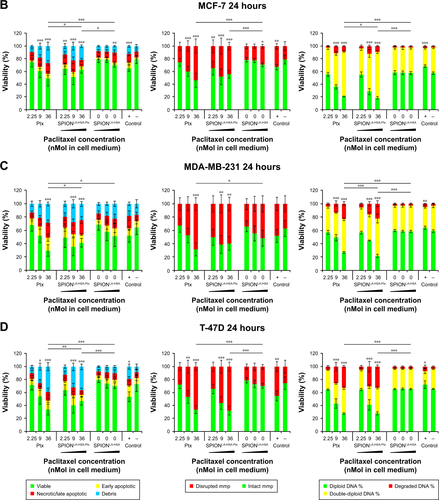
Figure S2 Viability of breast cancer cells 24 hours after Ptx treatment.
Notes: (A) BT-474, (B) MCF-7, (C) MDA-MB-231, and (D) T-47D cells were incubated for 24 hours with increasing amounts of free Ptx, SPIONLA-HSA-Ptx, and SPIONLA-HSA and analyzed by multiparameter flow cytometry. Viability was determined by AxV–FITC and PI staining (first column), yielding the percentage of viable (Ax− PI−), apoptotic (Ax+ PI−), and necrotic (PI+) cells. The status of the mitochondrial membrane potential was analyzed by DiIC1(5) staining and distinguished cells with intact (DiIC1(5) positive) and depolarized (DiIC1(5) negative) membranes (middle column). DNA degradation and cell cycle were determined by PIT staining and showed the amount of degraded DNA, diploid DNA (G1 phase), and double-diploid DNA (synthesis/G2 phase) (last column). Positive controls contain 2% DMSO, and negative controls represent the corresponding amount of solvent instead of drug or ferrofluid. Data are expressed as the mean ± SD (n=4 with technical triplicates). Statistical significance of viability, intact membrane potential, and diploid DNA content between control and samples are indicated with *P<0.01, **P<0.001, and ***P<0.0001, and were calculated via Student’s t-test analysis.
Abbreviations: AxV, Annexin A5; DiIC1(5), 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide; DMSO, dimethyl sulfoxide; FITC, fluorescein isothiocyanate; MMP, mitochondrial membrane potential; PI, propidium iodide; PIT, propidium iodide–Triton X-100; Ptx, paclitaxel; SPION, superparamagnetic iron oxide nanoparticles; SPIONLA-HSA, lauric acid- and human serum albumin-coated SPIONs; SPIONLA-HSA-Ptx, SPIONLA-HSA functionalized with paclitaxel.