Figures & data
Table 1 In Vivo Studies On The Liver Toxicity Of Metallic Nanoparticles (MNPs)
Table 2 In Vitro Studies On The Liver Toxicity Of Metallic Nanoparticles (MNPs)
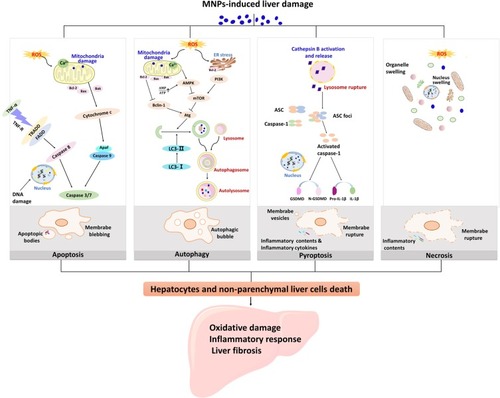
Figure 1 Different death mechanisms of liver cells are involved in the pathogenesis of liver injury induced by MNPs. Liver damage caused by MNPs is associated with oxidative damage, inflammatory response, and liver fibrosis in the liver. Apoptosis, autophagy, pyroptosis, and necrosis are all pathways of hepatocyte death. ROS induced by MNPs is responsible for the lipid peroxidation injury of the hepatic subcellular organelles. Apoptosis is considered as type I programmed cell death and mainly mediated by endogenous mitochondrial pathway and exogenous death receptor pathway. Mitochondrial ROS inhibited Bcl-2, and Fas-related death domain proteins (FADD) were activated, all of which eventually activated caspase 3 or caspase 7. Autophagy cell death is a programmed cell death different from apoptosis with initiation, nucleation of autophagosomes, phagosome expansion and completion, and autolysosome docking. Mitochondria and endoplasmic reticulum oxidative stress cause changes in the upstream molecules of autophagy and regulate autophagy-related (Atg) molecules. Pyroptosis is a form of inflammatory cell death that characterized by caspase-1-dependent formation of plasma membrane pores, and mainly manifested by lysosome rupture, ROS production and the activation of inflammation, leading to the release of pro-inflammatory cytokines and cell lysis. Necrosis is due to the production of ROS or instability of lysosome, release of calpain, and decrease of ATP level. The characteristics of necrosis include plasma membrane rupture, mitochondrial swelling, lysosome rupture, and intracellular contents release. Cell necrosis leads to inflammation that is not related to caspase cascade.