Figures & data
Figure 1 Physical properties and cellular uptake of CuONPs. (A) A representative transmission electron microscope (TEM) image of CuONPs dissolved in distilled water. (B) Representative FACS data for SSC intensity of HUVECs treated with 20 μg/mL CuONPs for 12 h. Mock, untreated HUVECs. Unpaired Student’s t-tests were performed for statistical analysis. **p < 0.05 versus untreated HUVECs. (C) Representative TEM images of HUVECs treated with 20 μg/mL CuONPs for 12 h. Yellow arrows indicate lysosomal deposition of CuONPs.
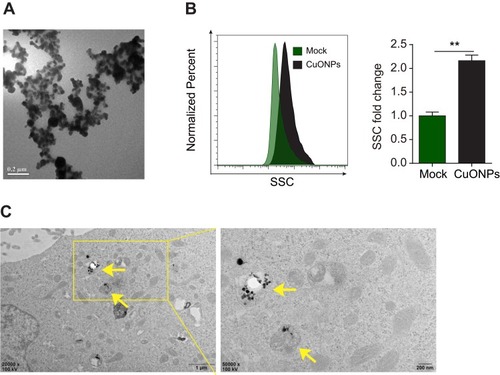
Figure 2 DNA damage and cell death in CuONPs-treated HUVECs. (A) MTS assay of HUVECs treated with different concentrations of CuONPs (0, 10, 20, or 40 μg/mL) for 24 h. (B) Representative FACS data for CuONPs-treated HUVECs after Calcein AM (live cell fluorescent probe) staining. (C) Comet assay to evaluate CuONPs-induced DNA damage in HUVECs. (D) Immunofluorescence assay of γH2AX foci formation in CuONPs-treated HUVECs. (E) Western blotting assay of phospho-ATR, phospho-ATM, phospho-p53 and phospho-H2AX (γH2AX) in HUVECs treated with CuONPs (20 μg/mL) for 0, 1, 3, 6 and 12 h. Actin was used as a loading control. In (A), one-way ANOVA followed by Tukey’s test was performed for statistical analysis. In (B), unpaired Student’s t-tests were performed for statistical analysis. **p < 0.05 versus untreated HUVECs.
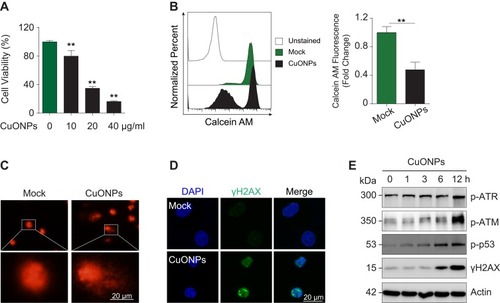
Figure 3 CuONPs treatment caused oxidative stress in HUVECs. (A) Representative FACS data for cellular superoxide anions in CuONPs-treated HUVECs after staining with the fluorescent probe DHE. (B) Representative FACS data for mitochondrial superoxide anions in CuONPs-treated HUVECs after staining with the fluorescent probe MitoSOX. (C) Western blotting analysis of protein levels of GCLM and HO-1. Actin was used as a loading control. (D) GSH/GSSG ratio assays of HUVECs treated with CuONPs (20 μg/mL) for 0, 1, 3, 6 and 12 h. (E) Lipid peroxidation (MDA) assay of HUVECs treated with CuONPs (20 μg/mL) for 0, 1, 3, 6 and 12 h. In (D) and (E), one-way ANOVA followed by Tukey’s test was performed for statistical analysis. **p < 0.05 versus untreated HUVECs.
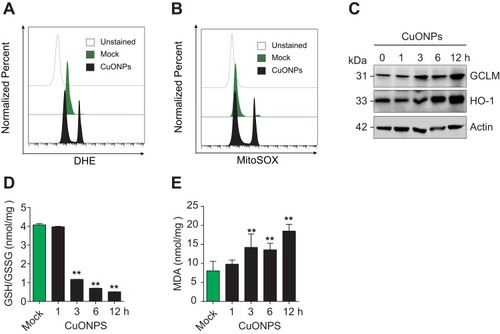
Figure 4 Oxidative stress-mediated DNA damage and cell death in CuONPs-treated HUVECs. (A) MTS assay of HUVEC cell viability after pretreatment with different concentrations of NAC (0, 5, 10 or 20 mM) for 1 h and then treatment with CuONPs (20 or 40 μg/mL) for 24 h. one-way ANOVA followed by Tukey’s test was performed for statistical analysis. **p < 0.05. (B) Representative FACS data for Calcein AM fluorescence of CuONPs-treated HUVECs. (C) Western blotting assay of phospho-ATR, phospho-ATM, phospho-p53 and γH2AX in HUVECs, which were pretreated with or without NAC (10 mM) for 1 h and then treated with CuONPs (20 μg/mL) for 12 h. Actin was used as a loading control. (D) Immunofluorescence assay of γH2AX foci formation in CuONPs-treated HUVECs.
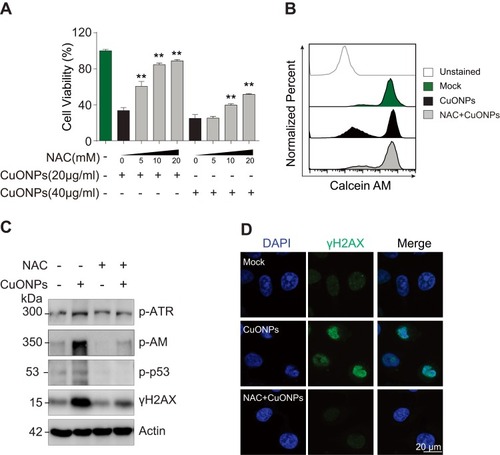
Figure 5 p38 MAPK was involved in DNA damage and cell death in CuONPs-treated HUVECs. (A) Western blotting assay of phospho-p38, phospho-Hsp27 and phosphor-ATF-2 in HUVECs treated with CuONPs (20 μg/mL) for 0, 1, 3, 6 and 12 h. Actin was used as a loading control. (B) Western blotting assay of p38α in HUVECs transfected with 75 nM siNC (negative control siRNA) or si-p38α for 48 h. Actin was used as a loading control. (C) Western blotting assay of phospho-ATR, γH2AX, phospho-Hsp27 and phospho-ATF-2 in HUVECs, which were transfected with siNC or si-p38α for 48 h and then treated with CuONPs (20 μg/mL) for 12 h. Actin was used as a loading control. (D) MTS assay of HUVEC after transfection with siNC or si-p38α for 48 h and then treatment with different concentrations of CuONPs (0, 10, 20 or 40 μg/mL) for 24 h. One-way ANOVA followed by Tukey’s test was performed for statistical analysis. **p < 0.05 versus untreated HUVECs.
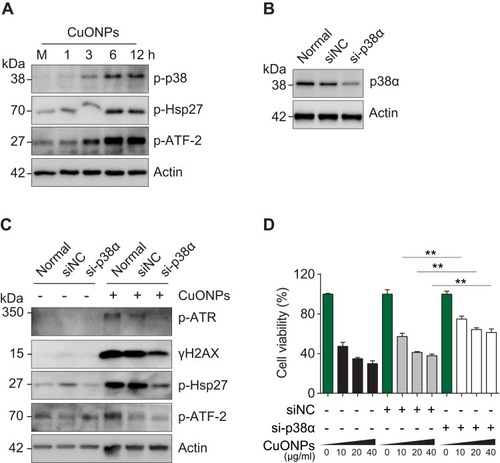
Figure 6 Copper ions chelator TTM alleviated HUVECs DNA damage and cell death induced by CuONPs. (A) and (B) Representative FACS data regarding cellular superoxide anions and mitochondrial superoxide anions after staining with the fluorescent probe DHE and MitoSOX, respectively. HUVECs were pretreated with different concentrations of the copper ions chelator TTM (0, 0.03 and 0.1 mM) for 1 h and then treated with CuONPs (20 μg/mL) for 12 h. (C) Lipid peroxidation (MDA) assay of HUVECs pretreated with or without TTM (0.1 mM) for 1 h and then treated with CuONPs (20 μg/mL) for 12 h. (D) Western blotting assay of phosphor-p38, phosphor-Hsp27 and phosphor-ATF-2 in HUVECs, pretreated with or without TTM (0, 0.03, and 0.1mM) for 1 h and then treated with CuONPs (20 μg/mL) for 12 h. Actin was used as a loading control. (E) Immunofluorescence assay of the level of γH2AX in CuONPs-treated HUVECs, pretreated with or without TTM (0.1 mM). (F) Western blotting assay of phospho-ATR, phospho-ATM, phospho-p53 and γH2AX in HUVECs, which were pretreated with or without TTM (0, 0.03, and 0.1 mM) for 1 h and then treated with CuONPs (20 μg/mL) for 12 h. Actin was used as a loading control. (G) MTS assay of HUVECs viability after pretreatment with different concentrations of the copper ions chelator TTM (0, 0.03, and 0.1 mM) for 1 h and then treatment with CuONPs (20 or 40 μg/mL) for 24 h. (H) Representative FACS data for Calcein AM fluorescence in HUVECs, which were pretreated with or without TTM (0.1 mM) for 1 h and then treated with CuONPs (20 μg/mL) for 12 h. In (C) and (G), one-way ANOVA followed by Tukey’s test was performed for statistical analysis. **p < 0.05.
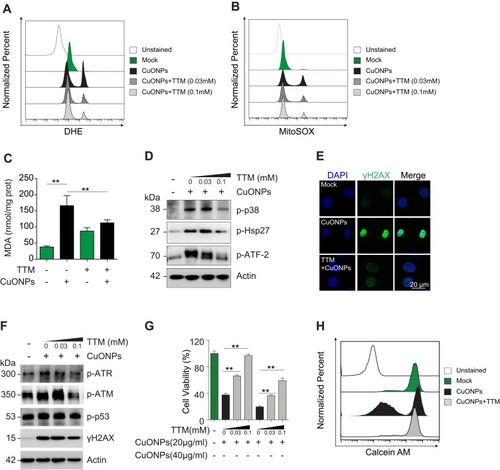
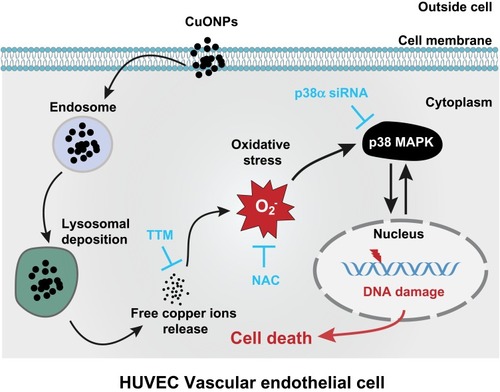
Figure 7 Schematic representation of the role of released copper ions in p38 MAPK-mediated DNA damage and cell death in CuONPs-treated HUVECs. Cellular uptake and lysosomal deposition of CuONPs lead to the release of copper ions from CuONPs, which triggers oxidative stress response and p38 MAPK signaling activation. The activation of p38 MAPK signaling pathway contributes to CuONPs-induced DNA damage and cell death in HUVECs. Meanwhile, CuONPs-induced DNA damage may feedback-regulate p38 MAPK signaling pathway, resulting in aggravation of DNA damage and cell death in HUVECs.