Figures & data
Figure 1 Top-down and bottom-up approaches for the synthesis of NPs. The top-down approach involves the transformation of bulk material by using energy to produce the powder form which is then transformed into smaller fragments with multiple layers and then to the monolayers leading to the formation of nanoparticles. On the other hand, the bottom-up approach uses the precursor molecules which are then ionized by using energy. Radicals, ions, and electrons thus produced are condensed to form clusters which are then transformed to nanoparticles.
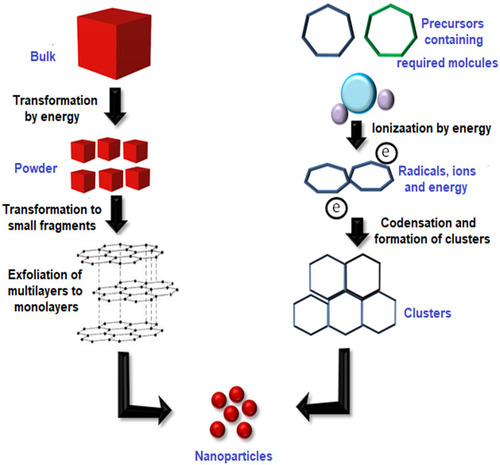
Figure 2 (A) Turkevich method for the synthesis of AuNPs. (B) Series of steps involved in the Burst method for the synthesis of AuNPs.
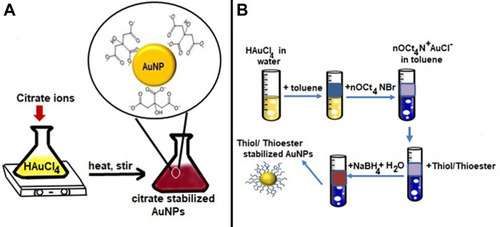
Figure 3 Series of steps involved in the synthesis of AuNPs. (A) Seed-mediated method. (B) Digestive ripening method.
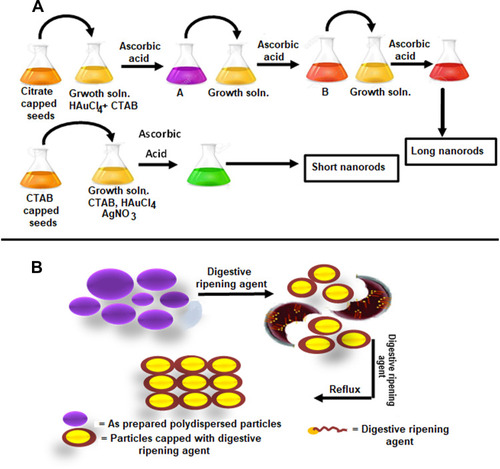
Table 1 Various Types of Living Organisms That Can Carry Either Intracellular or Extracellular Synthesis of AuNPs
Figure 4 (A) Electrostatic stabilization of gold nanoparticles. (B) Steric stabilization of gold nanoparticles.
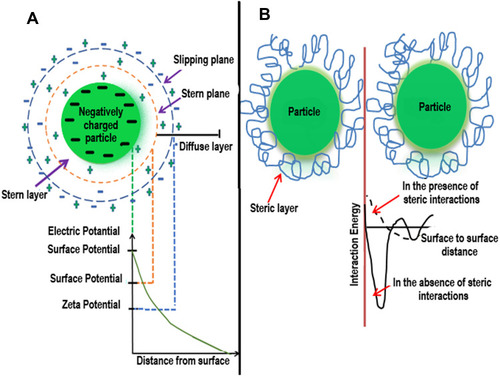
Figure 5 Unification of electrostatic and steric stabilization. Gold nanoparticles surround the ionic surfactants having polar ends and extended side chains. The area with high local concentration of stabilizer hinders the agglomeration of AuNPs.
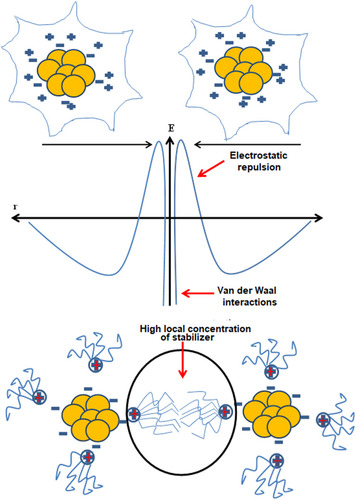
Figure 6 Synthesis of AuNPs of various shapes under different conditions due to different type of growths on the gold nuclei.
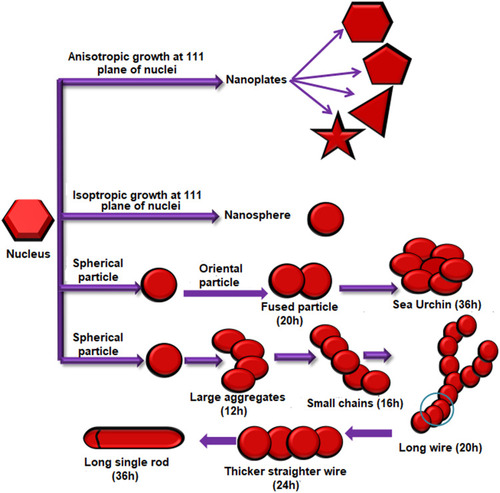
Table 2 List of Various Functional Groups, Their Ligands, and Key Features Which Make Them Suitable for Biological Applications
Figure 7 (A) Direct conjugation of AuNPs with methotrexate drug (MTX). Methotrexate molecule possesses two amine and carboxylic groups and exchanges citrate ions present on the surface of citrate capped AuNPs to form MXT-AuNPs. (B) Surface modification of AuNPs with PEG for conjugation of Doxorubicin (DOX) and Bleomycin (BLM). During the conjugation reaction, carboxylic groups on PEG forms amide bonds with amino groups present on BLM and DOX.
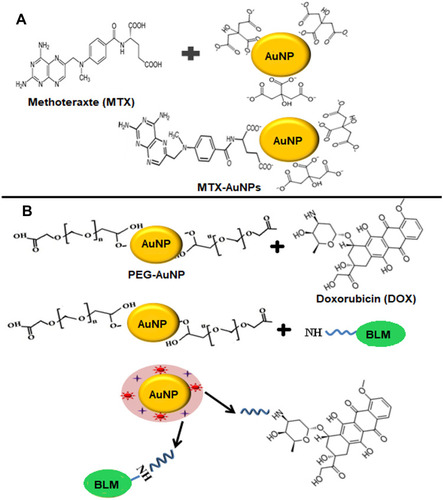
Figure 8 Surface modification of AuNPs with mercaptopropionic acid. These fAuNPs were conjugated with PEG- FITC (fluorescein isothiocyanate). These AuNPs entered the cell through endocytosis and were found localized in the endosomes and nucleus.
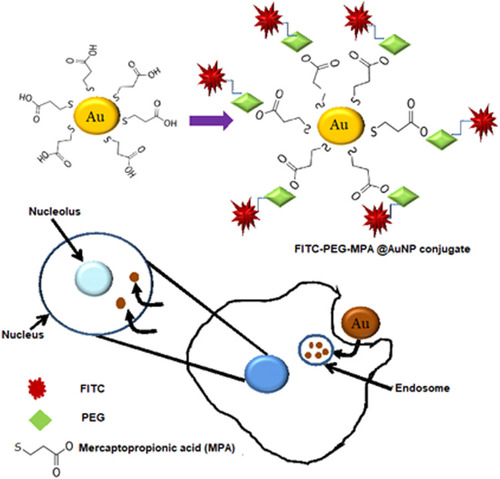
Figure 9 (A) Citrate capped AuNPs conjugated with mono/tetrathiol modified antisense oligonucleotides treated with DNAse. (B) Schematic illustration of preparation of insulin loaded AuNPs. Chitosan acts as a reducing and stabilizing agent during the formation of AuNPs. Insulin reacts with chitosan capped AuNPs through hydrogen bonding.
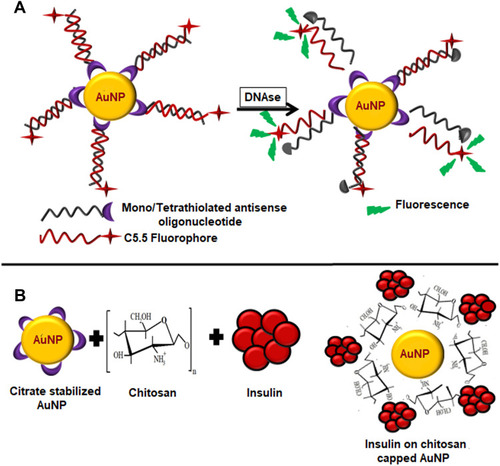
Figure 10 (A) Schematic Illustration of pH-mediated release of Doxorubicin from Doxorubicin conjugated AuNPs. First thiol stabilized AuNPs were prepared using Thiolated Methoxy PEG and Methyl Thioglycate (MTG). Doxorubicin was conjugated to AuNPs through hydrazone bond. The acidic conditions (pH 5.4) in the tumor cells cause the breaking of hydrazone bond releasing doxorubicin. (B) Glutathione-mediated release of a drug analog (HSBDP) from AuNPs. TTMA and HSBDP functionalized AuNPs when treated with glutathione at 37°C cause the release of HSBDP which can be detected by the fluorescence it produces in the free form which was quenched when conjugated with AuNPs.
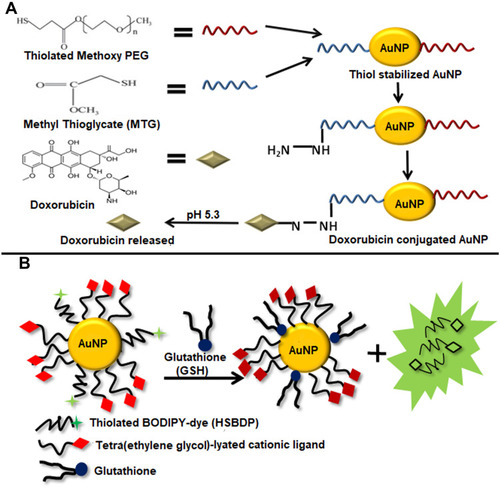
Figure 11 (A) Light-mediated drug release from AuNPs. Zwitterionic ligand integrates 5-fluorouracil (5-FU) to AuNPs through the Orthonitrobenzyl group which when irradiated with UV light with 365 nm wavelength undergoes photolytic cleavage of bond liberating 5-FU. (B) Release of singlet oxygen from phthalocyanines (Pc) conjugated AuNPs. When these conjugates were irradiated at 355 nm using a Q-switched Nd:YAG laser they generated singlet oxygen.
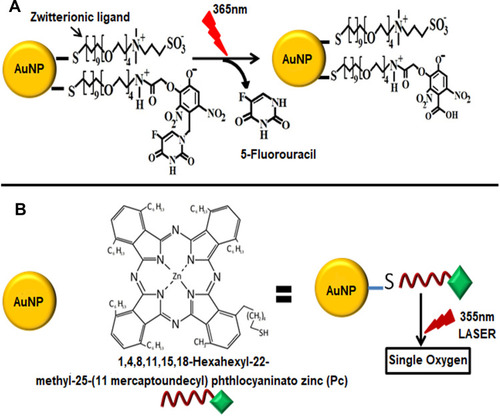
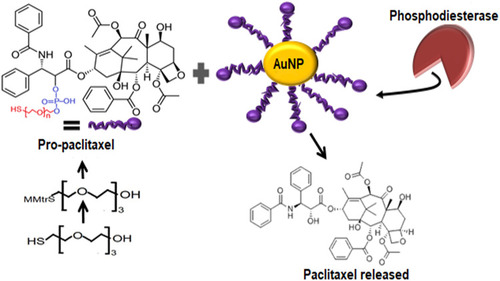
Figure 12 Enzyme-mediated drug release from AuNPs. Pro-paclitaxel was produced by first protecting the thiol group of tetraethylene glycol using (mono-4-methoxy) trityl chloride (MMTrCl) and then reacting it with paclitaxel. The thiol terminal of pro-paclitaxel incorporated it onto AuNPs. When treated with enzyme Phosphodiesterase, the Phosphodiester moieties were hydrolyzed causing the liberation of free paclitaxel.