Figures & data
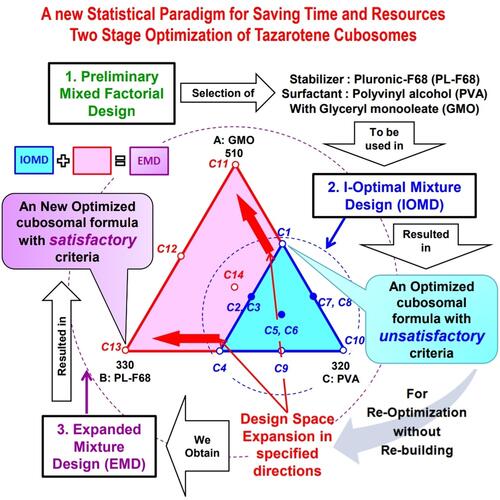
Table 1 Mixture Components Studied in the I-Optimal Mixture Design with Their Constraints Expressed in Different Values
Table 2 Composition and Characterization of Cubosomes Prepared According to Different Design Points of I-Optimal Mixture Design (C1-C10) and Extra Points Added in Expanded Design Space (C11–C14)
Figure 1 I-optimal mixture design (IOMD) space - defined by the constraints 300≤A≤420, 120≤B≤240 and 200≤C≤320 - and its DPs (C1–C11) together with extra added DPs (C11–C14) to give the expanded mixture design (EMD) space defined by the constraints 300≤A≤510, 120≤B≤330 and 110≤C≤320. Where; A = Glyceryl monooleate amount (mg), B = Pluronic F68 amount (mg), and C = Polyvinyl alcohol amount (mg). Design points (DPs): O single trial, ● replicated trial, ◊ selected optimized formulation from IOMD (IOMD-OF), Δ selected optimized formulation from EMD (EMD-OF).
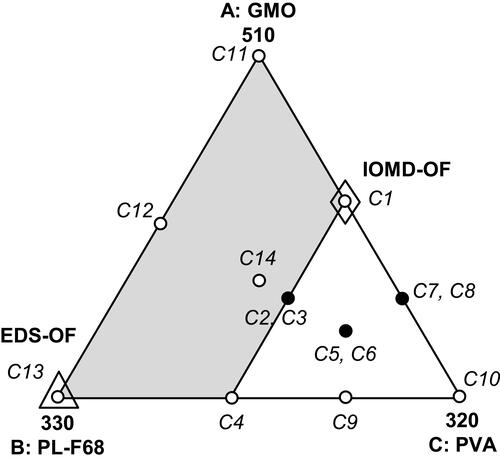
Figure 2 Release profiles of TAZA from trials (C1 – C14) compared with TAZA release from suspension.
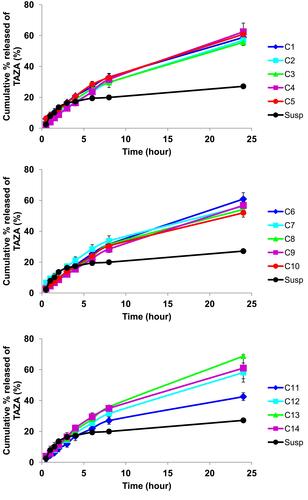
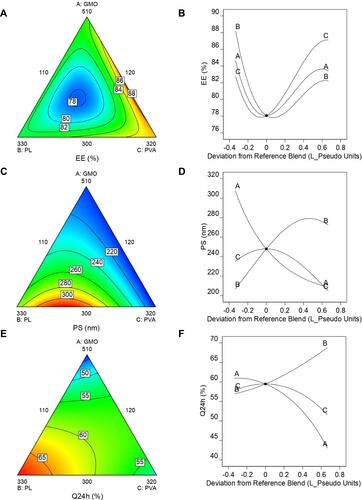
Figure 3 Contour plots showing the changes in (A) Entrapment efficiency, (B) Particle size, (C) Drug percent released after 24h (Q24h) and (D) desirability over the I-optimal mixture design space. Arrows represent regions of maximum: (C) Q24h and (D) desirability, ✖ represents the selected optimized formulation based on this design (IOMD-OF).
Table 3 Coefficient Estimates for Different Model Terms – Appearing in the Final Equations from the Expanded Mixture Design – for Each Response and Their Significance, Together with Models’ Types and Evaluation
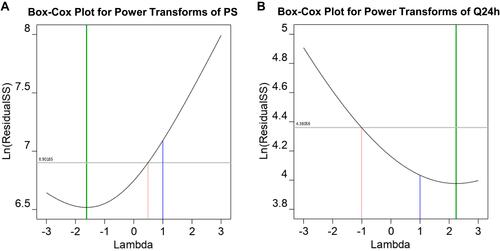
Figure 4 Box-Cox plot for data transformation showing ln residual sum of squares (ln residual SS) without transformation and after recommended transformation for the responses: (A) Particle size, (B) Drug percent released after 24h.