Figures & data
Figure 1 Effect of polymer size on pH-sensitive calcein release from large unilamellar vesicles.
Notes: Calcein release was measured by a calcein release assay, with calcein entrapped in large unilamellar vesicles and free polymers as described in the text. Liposomes were composed of egg phosphatidylcholine and cholesterol in a ratio of 60:40 (mol/mol) and calcein 100 mM. Free PEAA with a designed size was mixed with large unilamellar vesicles containing calcein at a polymer-to-lipid ratio of 10 μg/mg. The fluorescence intensity of the vesicles was measured after 20 minutes of incubation after acidification to pH 4.5.
Abbreviation: PEAA, poly(ethylacrylic acid).
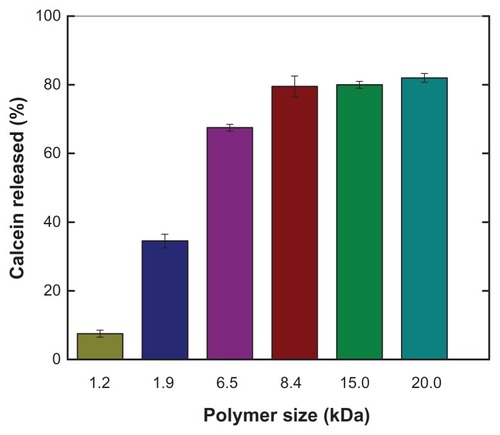
Figure 2 Characteristics of pH-sensitive PEAA vesicles with differing lipid composition by the post-insertion method. LUVs with encapsulated calcein 100 mM were prepared using a hydration extrusion method. PEAA-LUVs were then prepared by incubating lipo-PEAA with preformed LUVs at a polymer-to-lipid ratio of 20 μg/mg. (A) Percentage polymer insertion when different phospholipids were used to prepare the PEAA-liposomes. The noninserted polymer was removed, the liposomes were determined by radioactivity, and the polymer was quantified by fluorescence intensity. (B) Particle size of PEAA-LUVs. (C) Calcein released from PEAA-LUVs overnight at room temperature with incubation at neutral conditions (pH 7.4). Calcein release was measured as described in the text.
Abbreviations: DODAP, dioleoyl-1,2-diacyl-3-dimethylammonium-propane; DODAC, N,N-dioleyl-N,N-dimethylammonium chloride; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; PI, phosphatidylinositol; DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
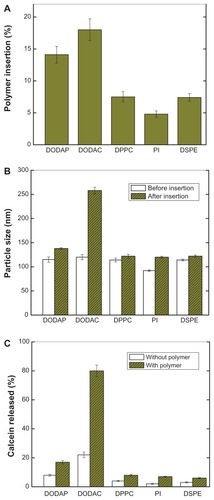
Figure 3 Effect of cholesterol content on pH-induced calcein release of PEAA-LUVs.
Notes: Preformed LUVs with DSPC and differing amounts of cholesterol were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. Calcein released from PEAA-LUVs was measured at room temperature (25°C) as described in the text.
Abbreviations: Chol, cholesterol; DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
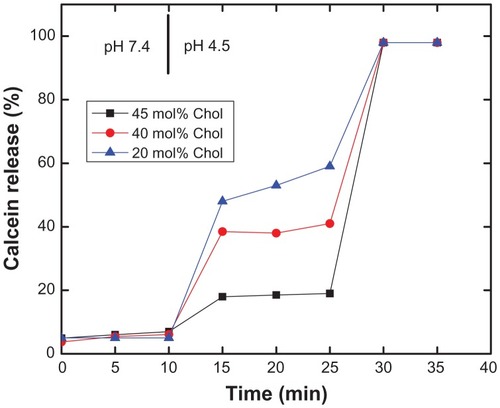
Figure 4 Effect of lipid chain length on pH-induced calcein release from PEAA-LUVs.
Notes: Preformed LUVs with differing cholesterol content (45 mol%, 40 mol%) were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-tolipid ratio of 20 μg/mg. Liposomal vesicles without PEAA inserted served as controls. The amount of calcein released from PEAA-LUVs was measured after 20 minutes of incubation at room temperature (25°C) as described in the text.
Abbreviations: Chol, cholesterol; DMPC, 1,2 dimyristoyl-sn-glycero-3-phosphocholine; DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
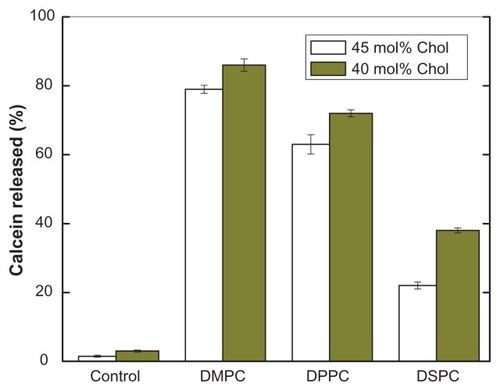
Figure 5 Effect of charged lipids on pH-induced release of calcein from PEAA-LUVs.
Notes: Preformed neutral LUVs (40 mol% cholesterol) or charged LUVs (40 mol% cholesterol, and 10 mol% charged lipid introduced) were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. The calcein released from the PEAA-LUVs was measured after 20 minutes of incubation at room temperature (25°C) as described in the text.
Abbreviations: DODAP, dioleoyl-1,2-diacyl-3-dimethylammonium-propane; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; PI, phosphatidylinositol; DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
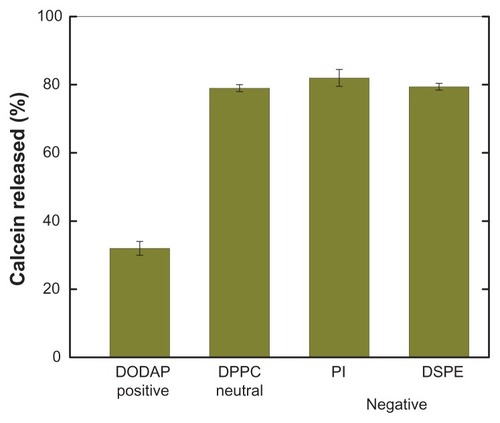
Figure 6 Effect of temperature on pH-induced release of calcein from PEAA-LUVs. Preformed LUVs with (A) a DSPC to cholesterol ratio of 60:40 (mol/mol) and (B) a DSPC to cholesterol ratio of 55:45 (mol/mol) was prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. Calcein released from PEAA-LUVs was measured at different temperatures as described in the text.
Abbreviations: DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
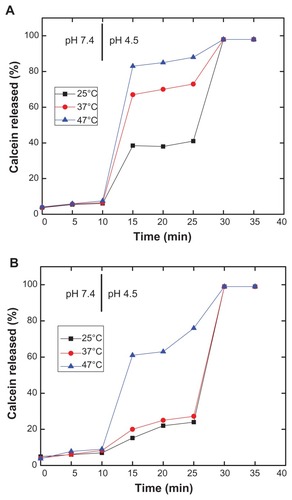
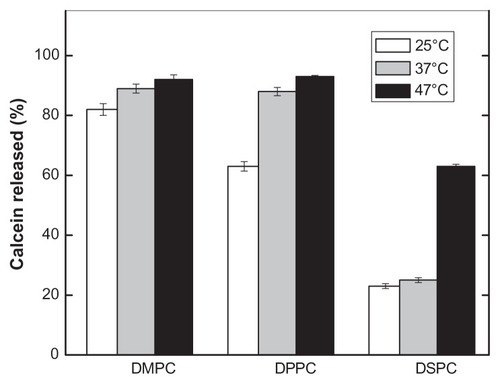
Figure 7 Effect of temperature on pH-induced calcein release of PEAA-LUVs containing different chain lengths of phosphocholine.
Notes: The preformed LUVs (45 mol% cholesterol) were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. The calcein released from PEAA-LUVs was measured after 20 minutes of incubation at differing temperatures as described in the text.
Abbreviations: DMPC, 1,2 dimyristoyl-sn-glycero-3-phosphocholine; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).