Figures & data
Figure 1 Schematic representation of the natural history of coronary atherosclerosis in US women.
Abbreviations: ET, estrogen therapy; HT, hormone therapy.
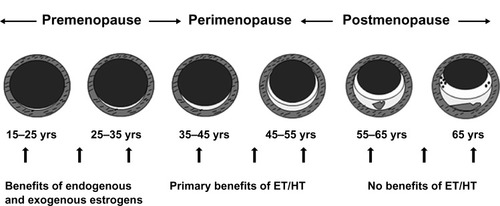
Table 1 Nonoral estrogen and progestogen products available in the US
Table 2 Oral estrogen and progestogen products available in the US
Table 3 Comparative effects of oral versus transdermal estrogens
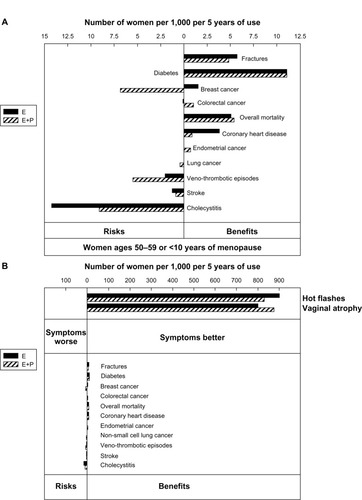
Figure 2 (A) Risks and benefits of MHT (expressed as attributable or excess risk) in women starting MHT between the ages of 50 and 59 years or less than 10 years after the start of menopause. Figure expanded from panel B for clear visualization. (B) Number of women expected to get hot flashes and vaginal dryness symptom benefit per 1,000 women taking MHT for 5 years. Design of panels A and B is the same. Panel B compares the number of women benefiting from relief of symptoms of hot flashes and vaginal atrophy with the number of women experiencing other risks and benefits.
Abbreviations: MHT, menopause hormone therapy; E, estrogen; E+P, estrogen + progestogen.