Figures & data
Table 1 Primary efficacy outcomesTable Footnote* used in two identically designed, double-blind, placebo-controlled, randomized controlled studies with estradiol valerate/dienogest, in women with heavy and/or prolonged menstrual bleeding without organic pathology
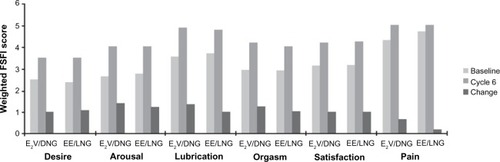
Figure 1 Bleeding reduction after 6 months of treatment with selected medical therapies, measured using the alkaline hematin method (data from randomized control trial unless otherwise stated).Citation15,Citation16,Citation47,Citation50,Citation58–Citation61
Abbreviations: DNG, dienogest; E2V, estradiol valerate; EE, ethinyl estradiol; LNG–IUS, levonorgestrel-releasing intrauterine system; LNG, levonorgestrel; MBL, menstrual blood loss; MPA, medroxyprogesterone acetate; TID, three times daily.
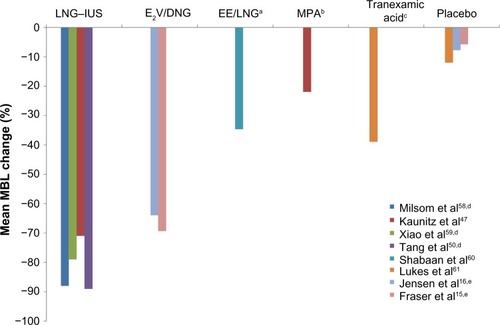
Figure 2 Change from baseline to cycle 6 in the average of the three highest VAS values on cycle days 22–28, for the hormone withdrawal-associated symptoms of headache or pelvic pain, in women who received treatment with E2V/DNG compared with (A) triphasic EE/NGM and (B) EE/LNG.
Abbreviations: DNG, dienogest; E2V, estradiol valerate; EE, ethinyl estradiol; LNG, levonorgestrel; NGM, norgestimate; SD, standard deviation; VAS, visual analog scale.
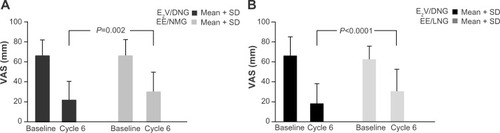
Figure 3 Scores of the Female Sexual Function Index subdomains, for E2V/DNG and EE/LNG, at baseline and cycle 6, and the change from baseline.
Abbreviations: DNG, dienogest; E2V, estradiol valerate; EE, ethinyl estradiol; FSFI, Female Sexual Function Index, LNG, levonorgestrel.