Figures & data
Figure 1 The adherence-aiding dispenser.
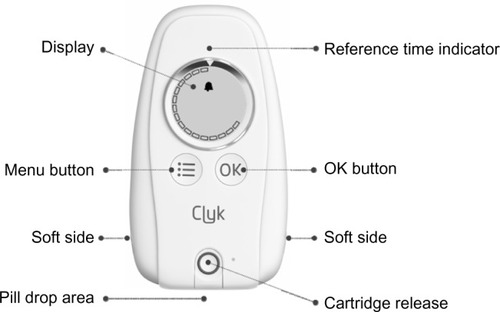
Figure 2 Disposition of subjects (FAS).
Abbreviation: FAS, full analysis set.
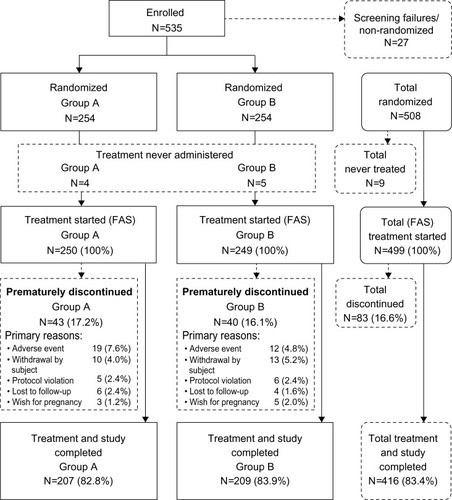
Table 1 Baseline demographics and clinical characteristics (full analysis set)
Figure 3 Box and whisker plot for number of missed pills per year in group A (activated acoustic alarm) and group B (deactivated acoustic alarm) according to dispenser (A) and diary card data (full analysis set) (B).
Abbreviation: Q, quartile.
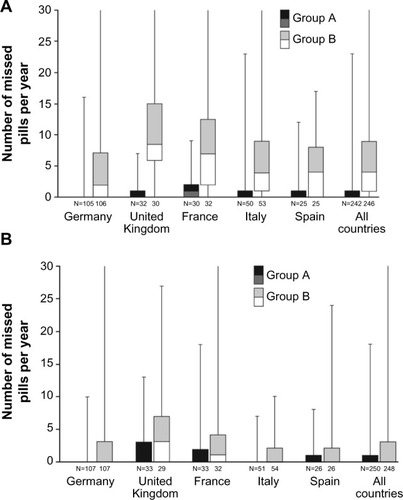
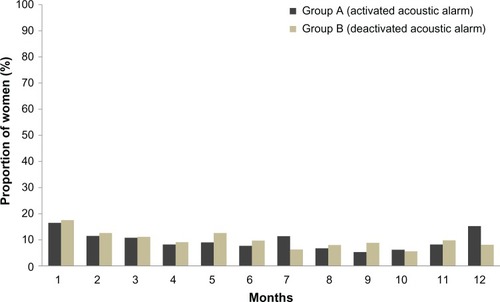
Figure 4 Proportion of women with missed pills over 1 year in group A (acoustic alarm activated) and group B (acoustic alarm deactivated) (full analysis set).
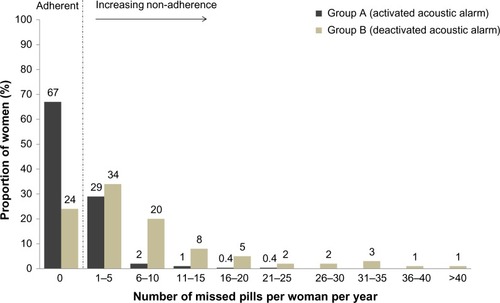
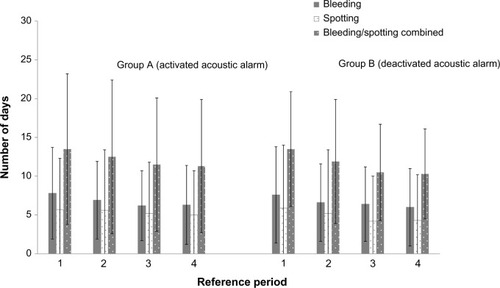
Figure 5 Mean (standard deviation [SD]) cycle length (A) and mean (SD) number of days with bleeding (excluding spotting), and bleeding and spotting combined over 1 year by country (B) (diary card data for pooled treatment groups; full analysis set).
![Figure 5 Mean (standard deviation [SD]) cycle length (A) and mean (SD) number of days with bleeding (excluding spotting), and bleeding and spotting combined over 1 year by country (B) (diary card data for pooled treatment groups; full analysis set).](/cms/asset/84d124c7-3f1d-4c1a-864a-f3a93452b294/djwh_a_71906_f0005_b.jpg)