Figures & data
Figure 1 Study design. Stable asthmatic patients received 8 weeks of fluticasone furoate/vilanterol (FF/VI) dry powder inhaler (DPI) (100/25 μg 1 puff once-daily) or budesonide/formoterol (BUD/FM) DPI (160/4.5 μg 2 puffs twice-daily) treatment. After a 4–8-week washout period, patients received another crossover treatment for 8 weeks. We assessed pulmonary function, the 5-item version asthma control questionnaire (ACQ5), the asthma control test (ACT), and fractional exhaled nitric oxide (FeNO) at baseline and after 8 weeks of treatment (week 8). The incidence of asthma exacerbation and an adherence barrier questionnaire (Ask-12 survey) were evaluated at week 8.
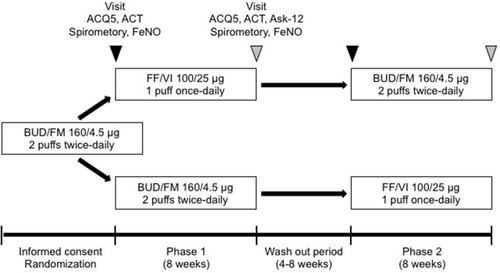
Table 1 Patient characteristics
Table 2 Patient treatment at the registration
Table 3 Incidence of asthma exacerbation
Table 4 Change in parameters from baseline to after 8 weeks of treatment
Table 5 Scoring of Ask-12 adherence barrier survey
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.

