Figures & data
Table 1 Baseline Demographic and Clinical Characteristics in Patients with Physician-Assessed Severe Asthma, including those with Uncontrolled Severe Asthma and Controlled Severe Asthma
Figure 1 (A) Box plot of mMRC dyspnea grade and (B) proportion of patients by mMRC dyspnea grade in patients with uncontrolled severe asthma and controlled severe asthma.
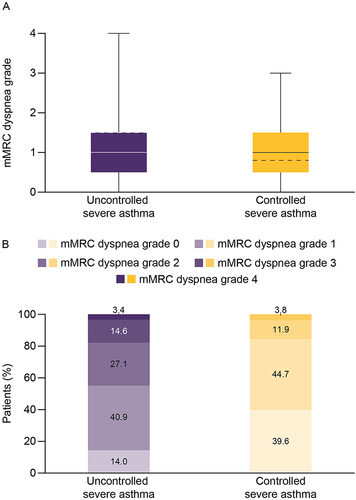
Figure 2 Box plot of RSQ total score in patients with uncontrolled severe asthma and controlled severe asthma.
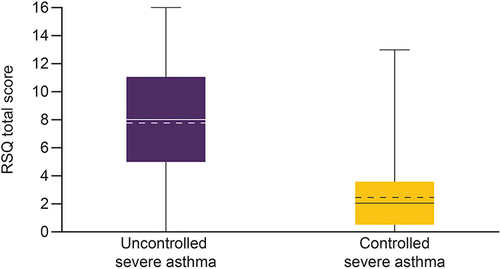
Figure 3 RSQ (A) shortness of breath, (B) limited activities, (C) rescue inhaler use, and (D) night awakening in patients with uncontrolled severe asthma and controlled severe asthma.
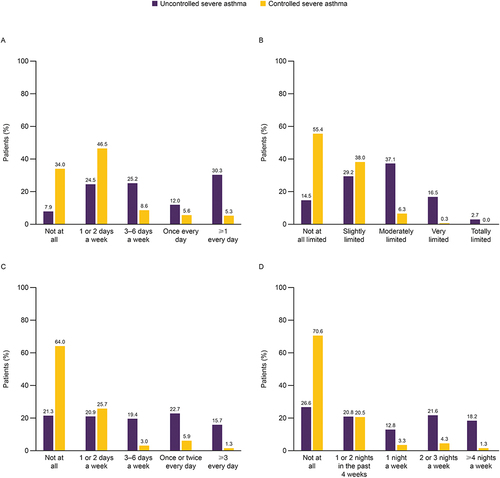
Figure 4 Box plot of ACT score in patients with uncontrolled severe asthma and controlled severe asthma.
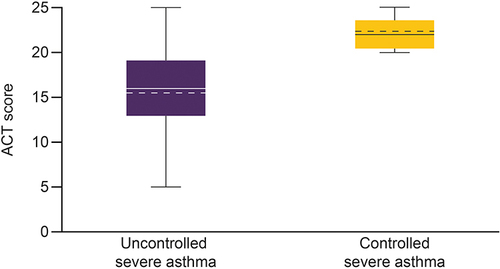
Figure 5 Box plots for (A) SGRQ total score, (B) CAAT score, (C) EQ-5D-5L index value, and (D) EQ-VAS score in patients with uncontrolled severe asthma and controlled severe asthma.
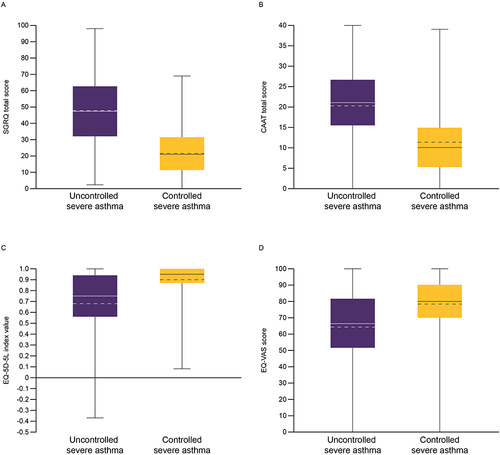
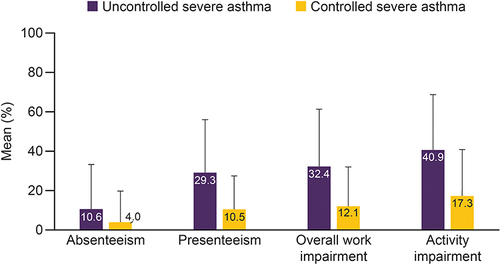
Figure 6 Productivity loss in patients with uncontrolled severe asthma and controlled severe asthma.