Figures & data
Table 1 Demographic and baseline characteristics
Figure 1 The number of dosing days resulting in RFBMs increased during the QD phase in patients treated with methylnaltrexone, and those increases were maintained into the PRN phase.
Abbreviations: PRN, treatment as needed; QD, once daily; RFBMs, rescue-free bowel movements.
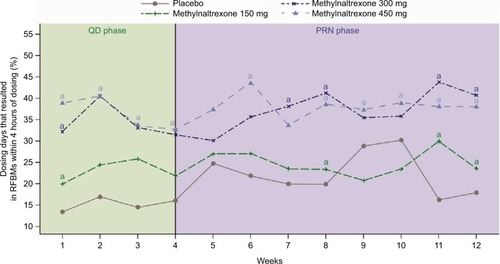
Figure 2 Time to achieve RFMB in patients with chronic noncancer pain and OIC receiving methadone was reduced by methylnaltrexone treatment.
Abbreviations: OIC, opioid-induced constipation; RFBM, rescue-free bowel movement.
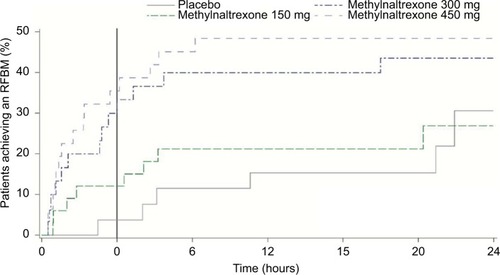
Figure 3 The percentage of patients responding to methylnaltrexone treatment in each treatment arm showed a trend for higher methylnaltrexone doses to increase the odds of responding.
Abbreviation: RFBM, rescue-free bowel movement.
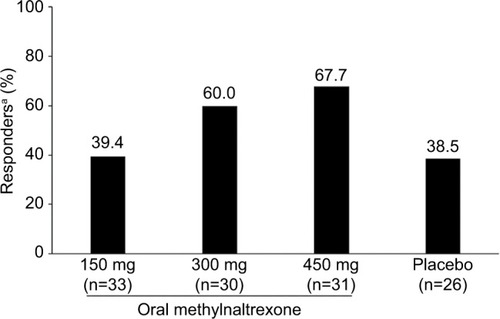
Table 2 AEsTable Footnotea in patients with chronic noncancer pain and OIC receiving methadone
