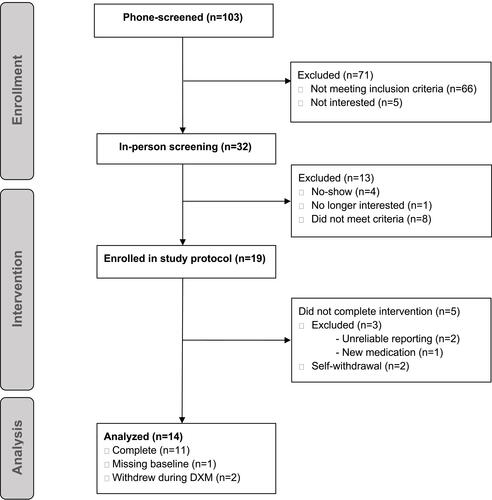
Figures & data
Table 1 Individual and Group-Level Illness Characteristics, Comorbid Medical Conditions, and Medications Reported in the Sample
Table 2 Individual and Group-Level Characteristics and Questionnaire Results from the Screening Visit
Table 3 Group Means and Standard Deviations on the Primary, Secondary, and Exploratory Treatment Outcomes (Raw Scores)
Figure 2 Individual changes in generalized pain ratings between the placebo and DXM conditions. The bold dashed line represents the change from mean placebo and mean DXM scores across participants.
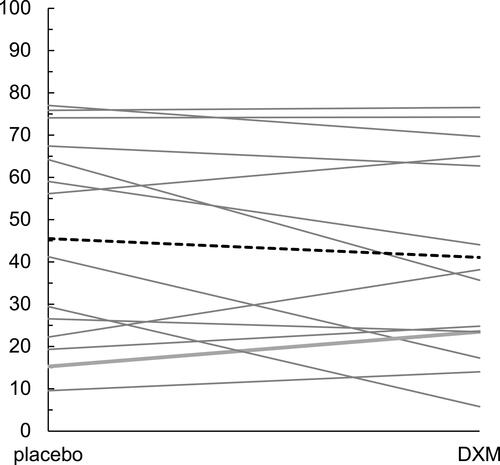
Table 4 GEE Model Estimates from Sensitivity Analyses Predicting Study Outcomes Over the Entire Treatment Period
Table 5 Adverse Event Occurrences During Placebo and DXM Treatment