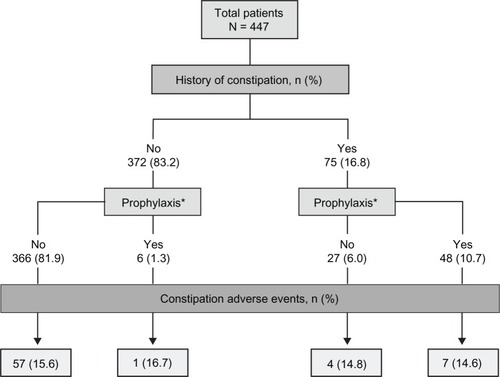
Figures & data
Figure 1 Study design, highlighting the conversion and titration phase.
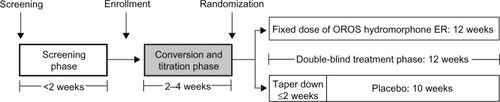
Table 1 Demographic and baseline characteristics
Table 2 Patients with response by prior opioid compound
Table 3 Duration of exposure
Figure 2 Distribution of initial and final doses of OROS hydromorphone ER in patients who achieved an effective dose.
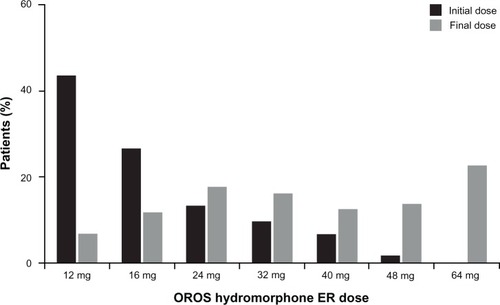
Table 4 Most common adverse events in the safety population (>5%) overall and according to achievement of a stable dose
Table 5 Summary of all AEs by OROS hydromorphone ER dose (safety population)