Figures & data
Figure 1 Patient disposition. Data is presented as n(%) of patients. The arrows indicate insufficient numbers.
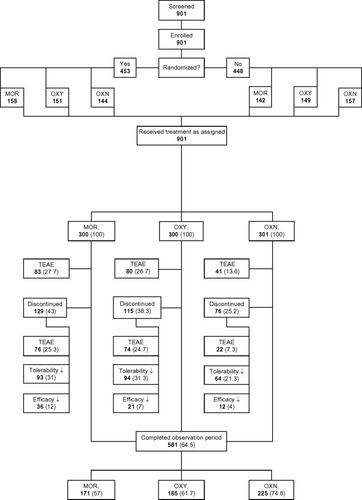
Table 1 Patient demographics and baseline characteristics
Figure 2 Change in mean BFI score during the course of the 12-week study.
Abbreviations: BFI, Bowel Function Index; BL, baseline; VAS100, 100 mm horizontal visual analog scale; W, treatment week.
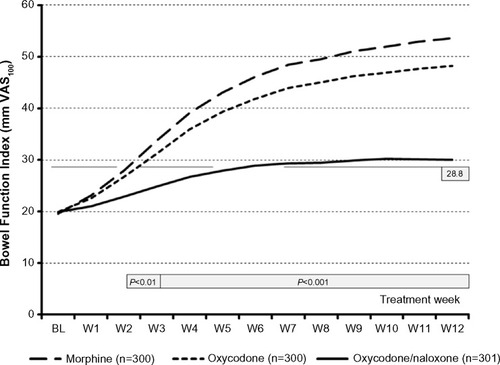
Table 2 Primary endpoint analysis
Figure 3 Proportion of patients who recorded a Bowel Function Index score above the normal range (≥28.8) (left), a significant worsening vs baseline (≥12 mm) (middle), and a CSBM ≥1 decrease vs baseline (right) at the end of a 12-week treatment with morphine (light grey), oxycodone (grey), and oxycodone/naloxone (dark grey).
Abbreviations: BFI, Bowel Function Index; CSBM, complete spontaneous bowel movement; VAS100, 100 mm horizontal visual analog scale.
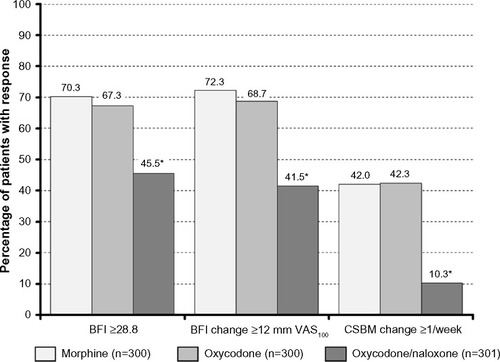
Table 3 Secondary tolerability analysis
Figure 4 Proportion of patients who recorded a ≥50% improvement (vs baseline) with respect to pain intensity (left), pain-related disabilities in daily life (middle), and quality of life (right) at the end of a 12-week treatment with morphine (light grey), oxycodone (grey), and oxycodone/naloxone (dark grey).
Abbreviation: QoL, quality of life.
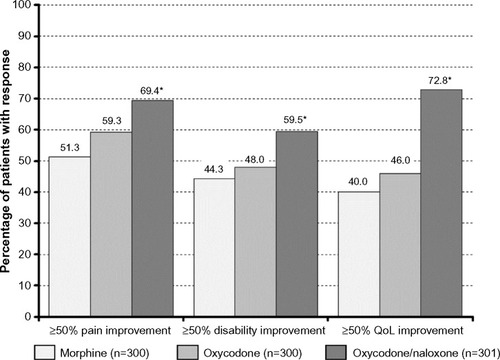
Figure 5 Distribution of time to onset of TEAEs reported for morphine (dashed line), oxycodone (dotted line), and oxycodone/naloxone (solid line) during a 12-week treatment period.
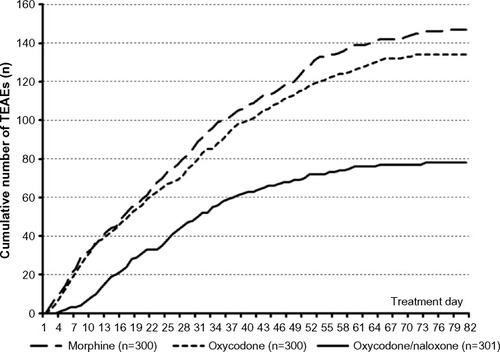
Figure 6 Number of TEAEs (left), patients affected by TEAEs (middle), and patients forced to discontinue treatment due to a TEAE (right), recorded during a 12-week treatment with morphine (light grey), oxycodone (grey), and oxycodone/naloxone (dark grey).
Abbreviation: TEAEs, treatment-emergent adverse events.
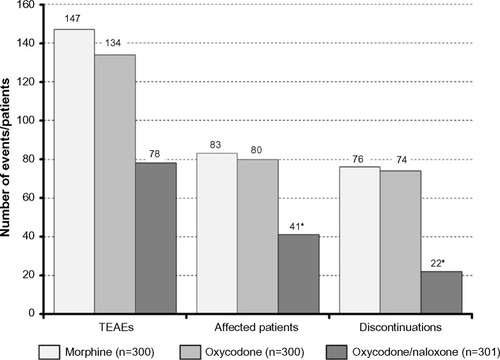
Table 4 Overall TEAE experience
