Figures & data
Table 1 Percentage of Double Positive Test (PROFILE1014 and ALEX) from Retrospective Analysis of Central Labs
Table 2 Comparison of Efficacy of Crizotinib (PROFEILE 1014) and Alectinib (ALEX) per Retrospective Analysis of Central Laboratory Analysis by FISH and IHC
Figure 1 Graphic depiction of the increasing capacities of successive generation of ALK TKI being developed including the anticipated capacities of a prototypic 5th-generation (5G) ALK TKI to overcome anticipated resistances to current approved or investigational ALK TKIs.
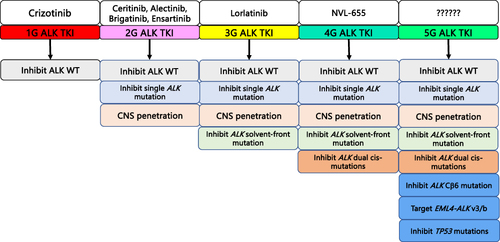
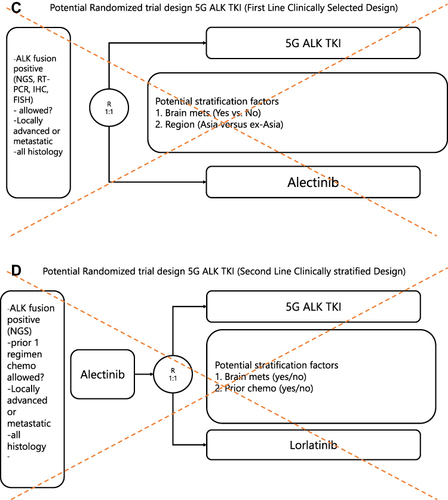
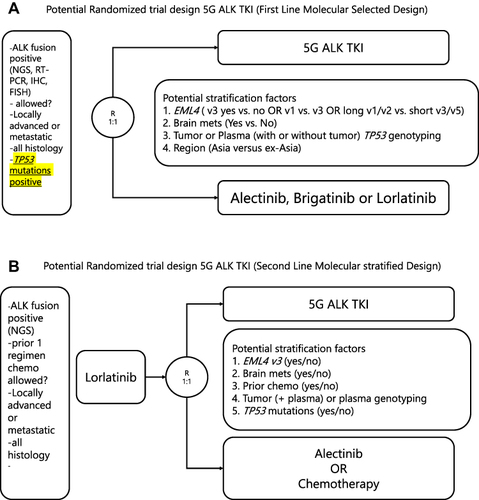
Figure 2 Hypothetical designs of pivotal randomized trials of a hypothetical 5th generation ALK TKI.