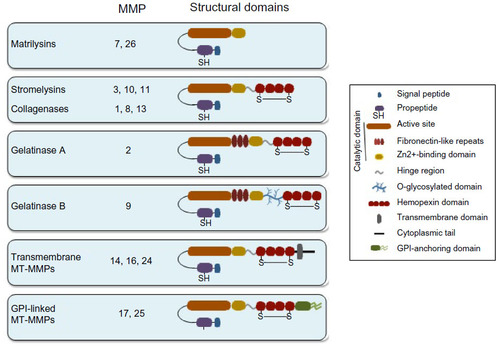
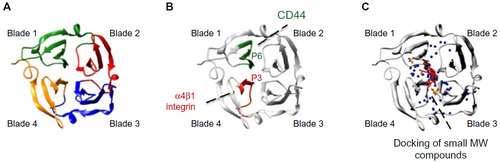
Notes: (
A) PEX9, similar to other PEX domains of MMPs, is composed of four structural blades surrounding a central cavity. (
B) Spatial location of the P3 and P6 amino acid sequences within PEX9 (research originally published in The Journal of Biological Chemistry. Ugarte-Berzal E, Bailón E, Amigo-Jiménez I, Albar JP, García-Marco JA, García-Pardo A. A novel CD44-binding peptide from the pro-matrix metalloproteinase-9 hemopexin domain impairs adhesion and migration of chronic lymphocytic leukemia (CLL) cells.
The Journal of Biological Chemistry. 2014; 289(22):15340–15349. © the American Society for Biochemistry and Molecular Biology.
Citation79). P3 is located in blade 4 and interacts with α4β1 integrin; P6 is located in blade 1 and binds to CD44.
Citation79 P3 and P6 act synergistically to inhibit MMP-9-induced CLL cell adhesion and migration, thus serving as therapeutic peptides and simultaneously pointing to “druggable” targets in PEX9. An additional CD44-binding sequence located in the outermost strand of blade 1 has also been reported. (
C) Diagram showing the binding sites within PEX9 of small MW compounds mapped by in silico docking (adapted from Cancer Research, Copyright 2011;71(14):4977–4988. Dufour A, Sampson NS, Li J, et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9, with permission from AACR
Citation82). As observed, both the small compounds and the P3 and P6 sequences are located in close proximity within the central cavity of PEX9. Targeting this region may therefore be a useful approach to control the pathogenic functions of MMP-9, particularly in cancer and inflammation.
Abbreviations: MW, molecular weight; MMP, matrix metalloproteinase; PEX, hemopexin domain; CLL, chronic lymphocytic leukemia.