Figures & data
Table 1 Summary of Physicochemical Features of NTB700 Nanoparticles
Figure 1 Synthesis, physicochemical characterization of NTB700 nanoparticles and pH reactivity. (A) Schematic representation of the synthesis of the core-shell silica nanoparticles, through a micelle-assisted method, where a surfactant is used to create a nanoreactor within which all reagents arrange. The base-catalysed hydrolysis of a silane precursor, along with different dyes functionalized with a trialkoxysilane group, able to covalently link to the silica matrix, leads to the formation of fluorescent monodisperse NPs. (B) Absorption (dotted grey line) and emission spectra (solid red line) of NTB700 nanoparticles. (C) NTB700 diluted 1:1000 analysed using a nanoparticle tracking analysis (NTA), results are represented as mean ± sd; n = 5. (D) Transmission electron microscopy (TEM) analysis of NTB700 diluted 1:100 (above) and 1:1000 (below), insert are enlargements of NP aggregates. Scale bar = 500 nm; insert scale bar = 100 nm. (E) NTB700 diluted 1:100 in buffers at different pH (2.5, 4.5, 5.5 and 7.2) and analysed by flow cytometry at three time points (0, 30 and 240 min) for FSC increase to investigate the presence of aggregates.
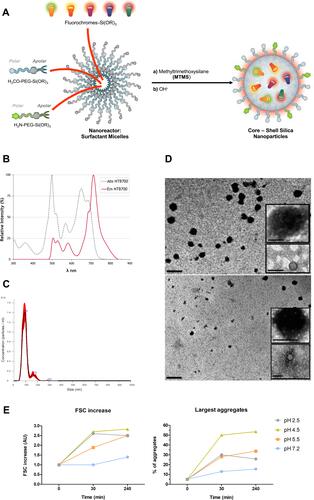
Figure 2 Kinetics NTB700 nanoparticle uptake in U937 cells. (A) U937 were incubated with 64 µg/mL NTB700 at several times (0, 30, 60 and 120 min). Dot plots (FSC vs. SSC) representing U937 treated with 64 µg/mL NTB700 nanoparticles during time. (B) FSC slightly decreased during time (above); cellular granularity (SSC values) increased in a time-dependent manner (below). (C) Time-dependence of NTB700 nanoparticle uptake in U937 cells. Mean Fluorescence Intensity (MFI) from the flow cytometry histograms is shown as a function of time. (D) Flow cytometry histogram overlay for cells treated for 1 h with different concentrations of NTB700 nanoparticles (0, 6.4, 64 and 640 µg/mL) (E) Cell viability of U937 loaded with increasing doses of NTB700 nanoparticles (0, 6.4, 64 and 640 µg/mL) determined by membrane permeability assay using Propidium Iodide (PI) and flow cytometry analysis to calculate the percentage of PI positive cells, indicating cell death. (F) U937 incubated for 1 h at 37°C with NTB700 nanoparticles (64 µg/mL), turned out best experimental conditions. Representative confocal images of Ctrl and NTB700 NPs-treated U937 cells. NTB700 in red. Scale bar = 10 µm. At least 10,000 events were analysed by flow cytometry for each experimental condition.
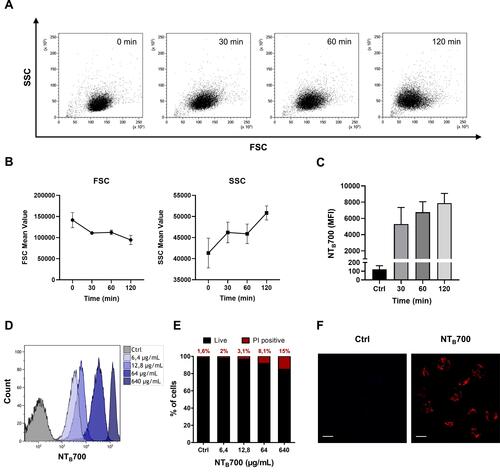
Figure 3 Effect of temperature and energy depletion on NTB700 nanoparticle internalization in U937. Cells were incubated with NTB700 (64 µg/mL) under different condition: control (NPs incubated at 37°C), +4°C and sodium azide (NaN3). The percentage of nanoparticle uptake was measured by MFI, determined through flow cytometry (2 independent replicas of 3 experiments), normalized with NP MFI values in normal condition at 37°C (Ctrl is 100% uptake). Asterisks denote a statistically significant difference effect of temperature and energy with their respective control (***p < 0.001, ****p = < 0.0001). At least 10,000 events were analysed by flow cytometry for each experimental condition.
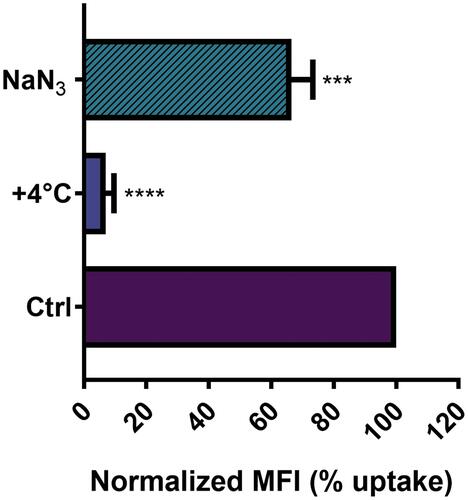
Table 2 Uptake Pathways and Inhibition Conditions
Figure 4 Effect of pharmacological inhibitors on cell viability and NTB700 endocytosis in U937 cells. (A) Cell viability assay performed through propidium iodide (PI) staining, analysed by flow cytometry, after 2 h 30 min of exposure to each one of the different inhibitors. Percentage of PI positive cells was calculated respect to control (untreated). (B) Effect of inhibitors on NTB700 internalization in U937. Cells were pre-treated with each one of the inhibitors (chlorpromazine, genistein, amiloride, nocodazole) for 30 min, followed by 2 h of exposure to NPs, in the presence of the same inhibitor, then washed and analysed by flow cytometry. Normalized MFI (mean fluorescence intensity) was calculated compared to “NPs” which is reported as 100% uptake (cells incubated with NPs in normal condition, at 37°C without inhibitors). Mean values and sd of 2 independent replicas of 3 experiments. Asterisks denote a statistically significant difference (*p < 0.05) between nocodazole and NPs. At least 10,000 events were analysed by flow cytometry for each experimental condition.
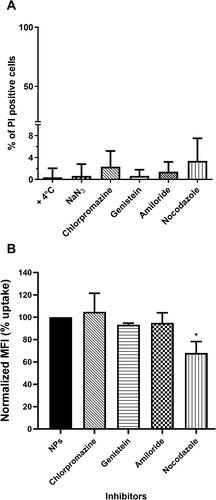
Figure 5 Lysosomes and mitochondria involvement in NTB700 uptake in U937 cells. (A) Representative confocal images of lysosomal (above) and mitochondria (below) involvement in U937 cells incubated for 1 h at 37°C with NTB700 (64 µg/mL) and then stained with LTG and TMRE. Figure shows LTG (green), TMRE (blue), NTB700 nanoparticles (red) and merged images (on the right); orange and violet indicate colocalization. Scale bars: 10 µm. Insert: binary version of merged images. The colocalization mask was generated by Image J software to show colocalization pixel. (B) Pearson’s colocalization coefficient (PCC) of LTG and TMRE with NTB700 in U937 cells. Pearson’s coefficients were derived from three completely independent experiments with >10 fields per experiment contributing to the cumulative result. Each value is expressed as PCC ± sd; asterisks denote a statistically significant difference (****p < 0.0001) between strains. (C) Flow cytometry analysis of lysosomes (LTG), mitochondria (MTG), ROS production (DCF) and autophagolysosomes formation (MDC) expressed as fold of change calculated as the ratio between cells incubated with NPs (64 µg/mL) and control cells. 1 value indicates no differences between treated and untreated. (D) Flow cytometry analysis of O2- production (MitoSOX) expressed as fold of change calculated as the ratio between cells incubated with NPs (64 µg/mL) and control cells at different time points (1, 4 and 24 h). Asterisks denote a statistically significant difference (***p < 0.001) between strains. At least 10,000 events were analysed by flow cytometry for each experimental condition.
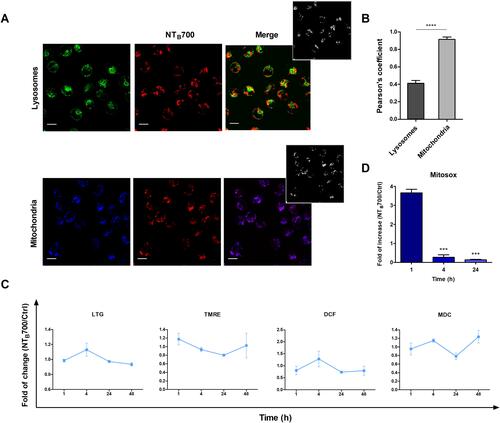
Figure 6 Kinetics of NTB700 uptake and confocal images of NP internalized in peripheral blood mononuclear cells (PBMCs). (A) PBMCs were incubated at 37°C with 64 µg/mL NTB700 at different time points (1, 4, 24 and 48 h). Lymphocyte (red) and monocyte (light blue) populations were selected through a gating strategy on FSC vs. SSC dot plot (Figure S1). Mean Fluorescence Intensity (MFI) from the flow cytometry histograms is shown as a function of time in hours. (B) PBMCs were incubated at 37°C with 64 µg/mL NTB700 at different time points (1, 4 and 24 h), washed and then stained with several mAbs to identify four PBMC subsets: CD5+ (T lymphocytes), CD19+ (B lymphocytes), CD16+ (NK cells) and CD14+ (monocytes). Asterisks denote a statistically significant difference (*p < 0.05) between 24 h strains. (C) Light and fluorescent images of PBMCs incubated for 1 h at 37°C with NTB700 nanoparticles (64 µg/mL). Representative confocal images of NTB700 nanoparticles uptake in PBMCs: brightfield (on the left), fluorescent nanoparticles inside cells (in the middle) and merged images (on the right). NTB700 in red. Scale bar = 10 µm. At least 10,000 events were analysed by flow cytometry for each experimental condition. Error bars represent sd.
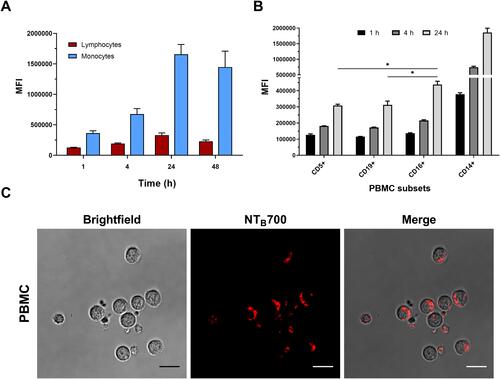
Figure 7 Assessment of lysosomes and mitochondria involvement in NTB700 uptake in PBMCs. (A) Pearson’s colocalization coefficient (PCC) of lysosomes (LTG) and mitochondria (TMRE) with NTB700 in PBMC cells. Pearson’s coefficients were derived from three completely independent experiments with >10 fields per experiment contributing to the cumulative result. Each value is expressed as PCC ± sd; (***p < 0.001). Below representative confocal images of lysosome (left) and mitochondria (right) colocalization in PBMC cells incubated for 1 h at 37°C with NTB700 nanoparticles (64 µg/mL) and then stained with LTG and TMRE. Figure shows LTG (green), TMRE (blue), NTB700 nanoparticles (red) and, respectively, orange and violet indicate colocalization. (B) Flow cytometry analysis of PBMCs incubated at 37°C with 64 µg/mL NTB700 at different time points (1, 4, 24 and 48 h) washed and then stained LTG, TMRE, DCF and MDC. Fold of change, calculated as the ratio between cells incubated with NPs and control cells (w/o NPs), from the flow cytometry histograms is shown as a function of time in hours. 1 value indicates no differences between treated and untreated. (C) Flow cytometry analysis of O2- production (MitoSOX) expressed as fold of change calculated as the ratio between cells incubated with NPs (64 µg/mL) and control cells at different time points (1, 4 and 24 h). Mean values and sd of 2 independent replicas of 3 experiments. Asterisks denote a statistically significant difference (***p < 0.001) between strains. (D) Effect of temperature and energy depletion on NTB700 nanoparticle internalization in PBMC. Cells were incubated with NTB700 (64 µg/mL) under different condition: control (NPs incubated at 37°C), +4°C and sodium azide (NaN3). The percentage of nanoparticle uptake was measured by MFI, determined through flow cytometry (2 independent replicas of 3 experiments), normalized with NP MFI values in normal condition at 37°C (Ctrl is 100% uptake). In red (on the left) lymphocyte data and in blue/light blue (on the right) monocyte data. Asterisks denote a statistically significant difference (***p < 0.001; ****p < 0.001) compared to control. At least 10,000 events were analysed by flow cytometry for each experimental condition.
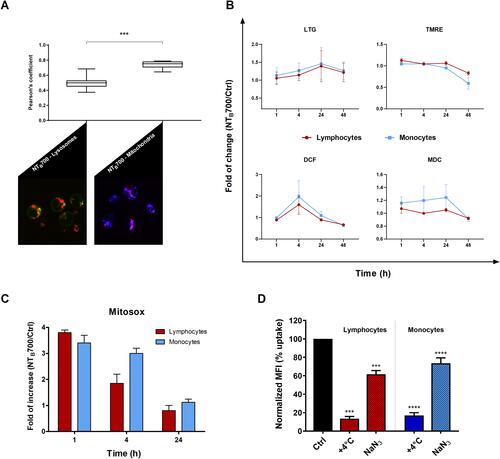
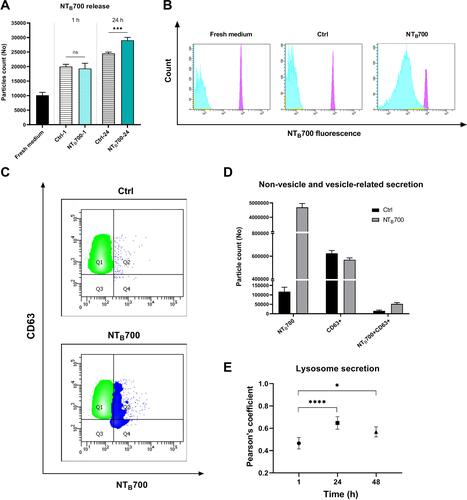
Figure 8 NTB700 release studies in U937 and PBMC. U937 were incubated with NTB700 (64 µg/mL) for 1 h, harvested and then seeded again with fresh medium (NP-free). The supernatants of all conditions (treatment of 1 h and 24 h after incubation in fresh medium following 1 h of NTB700 treatment) have been analysed by flow cytometry. (A) Histogram shows the quantification of extracellular vesicles (EVs) counted by using Dako CytoCountTM beads in fresh medium and released by the cells in 1 h of treatment or after 24 h of incubation in fresh medium following 1 h of NTB700 treatment or control cells, in the same experimental conditions. Asterisks denote a statistically significant difference (***p < 0.001) between 24 h strains. (B) Flow cytometry histograms showing the fluorescence of particles in NTB700 channel (violet peak represents Dako Cyto CountTM beads, light blue peak represents particles released based on gates in FSC vs SSC dot plot). Cells incubated with NPs presented a marked peak in NTB700 fluorescence channel (histogram on the right) compared to fresh medium and control cells (without NPs), which showed the ability of cells to excrete NPs after their internalization. (C) Flow cytometry contouring plots (fluorescence of particles in NTB700 channel vs. CD63 fluorescence) of supernatants of control (above) and treated cells (below) after 24 h in fresh medium, presenting in green CD63+ particles and in blue NTB700+particles. (D) Non-vesicle and vesicle-related secretion. PBMCs were incubated with NTB700 (64 µg/mL) for 1 h, harvested and then seeded again with fresh medium (NP-free). The supernatants have been analysed by flow cytometry. Histogram shows the quantification of extracellular vesicles (EVs) counted by using Dako CytoCountTM beads and CD63+ particles released by the cells after 24 h of incubation in fresh medium following 1 h of NTB700 treatment or control cells in the same experimental conditions w/o NPs. (E) Pearson’s colocalization coefficient (PCC) of LTG with NTB700 in PBMC varying during time to investigate lysosome particle secretion. Pearson’s coefficients were derived from three completely independent experiments with >10 fields per experiment contributing to the cumulative result. Each value is expressed as PCC ± sd; Asterisks denote a statistically significant difference (*p < 0.5, ****p < 0.0001) between strains. At least 50,000 events were analysed by flow cytometry for each experimental condition.