Figures & data
Figure 1 Range of American College of Rheumatology responder rates from different clinical studies of leflunomide. Data at six months and one year are taken from double-blind randomized placebo and active comparator-controlled clinical trials.Citation8,Citation24,Citation25 Patients completing 12 months were re-enrolled to a year 2 cohort and remained blinded to treatment allocation.Citation33–Citation35 Those completing two years were eligible to enroll in an open-label, non-controlled extension study to complete five years of treatment.Citation37
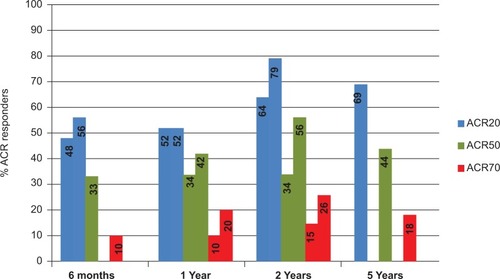
Figure 2 Sustained DAS28 response over time for leflunomide 20 mg daily following a loading dose of 100 mg daily for three days (percentage of patients with a response maintained to 24 weeks). Data from the RELIEF study.Citation51
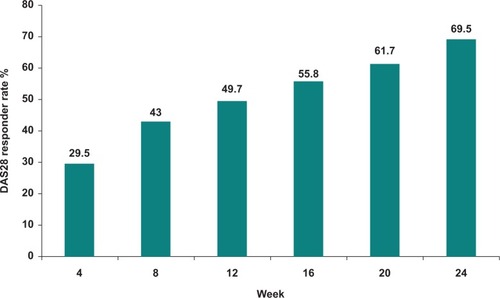
Figure 3 Quality of life changes in randomized controlled trials of LEF versus MTX over two years assessed by Medical Outcomes Survey Short Form 36 (SF-36). Vertical bars show baseline and 24-month data for each domain of the SF-36, dashed horizontal lines show US population norms.Citation143
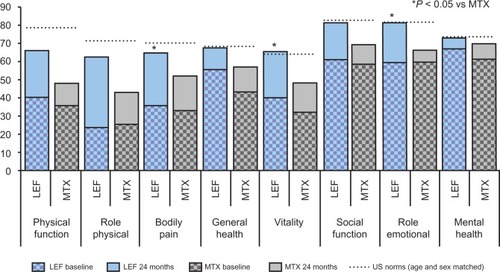
Figure 4 American College of Rheumatology response rates for combination therapy of leflunomide plus methotrexate versus methotrexate plus placebo.Citation49
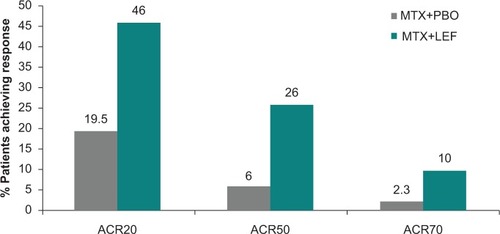
Figure 5 Change in quality of life occurring over time by treatment allocation with biologic DMARDs in combination with methotrexate or leflunomide.Citation76
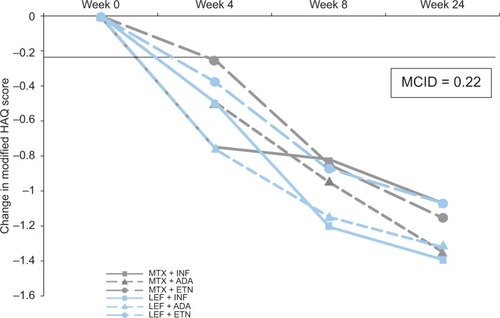
Table 1 Management strategies for side effects of leflunomide