Figures & data
Table 1 Patient baseline characteristics
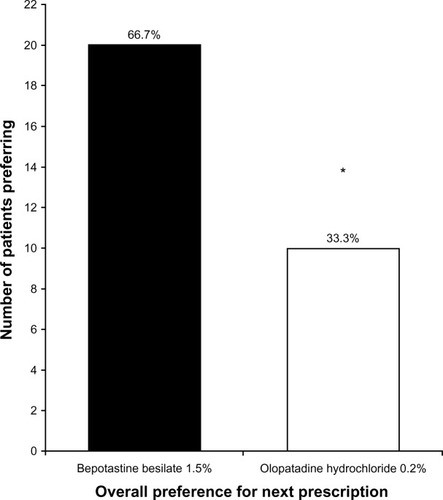
Figure 1 (A) Morning and evening relief of ocular itch over 14 days of treatment (mean ± standard deviation). Ocular itch was graded on a 1–5 scale, with 5 being completely relieved. *P = 0.011 versus bepotastine besilate 1.5% in the evening; †P < 0.0001 versus olopatadine hydrochloride 0.2% in the morning. (B) Patient preference for all-day relief of ocular itching at study end (visit 3, day 35).
Abbreviation: SD, standard deviation.
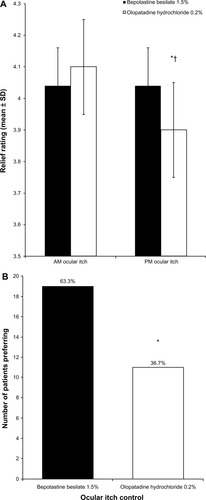
Figure 2 (A) Morning and evening relief of itchy/runny nose over 14 days of treatment (mean ± standard deviation). Nasal symptoms were graded on a 1–5 scale, with 5 being completely relieved. *P < 0.0001 versus bepotastine besilate 1.5% in the morning; **P < 0.0001 versus bepotastine besilate 1.5% in the evening; †P = 0.035 versus bepotastine besilate 1.5% in the morning. (B) Patient preference for all-day relief of itchy/runny nose at study end (visit 3, day 35).
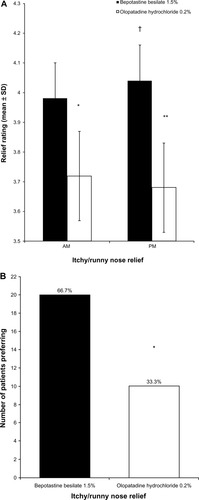
Figure 3 (A) Evening eye drop comfort over 14 days of treatment (mean ± standard deviation). Eye drop comfort was graded on a 1–5 scale, with 5 being very comfortable. (B) Patient preference for eye drop comfort at study end (visit 3, day 35).
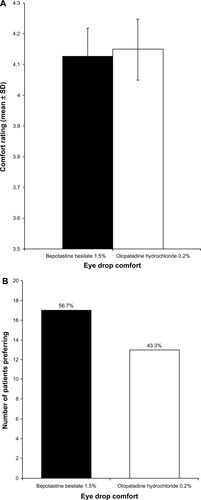
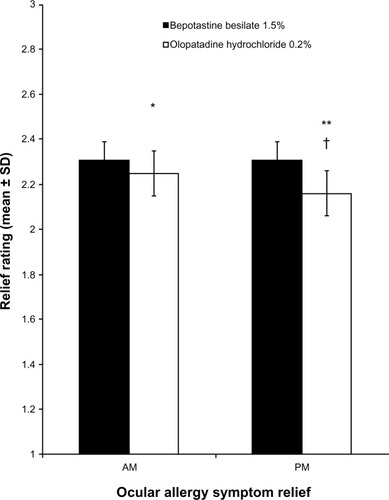
Figure 4 Morning and afternoon/evening relief of overall ocular allergy symptoms over 14 days of treatment (mean ± standard deviation).