Figures & data
Figure 1 Flow diagram of study selection.
Abbreviations: CENTRAL, Cochrane Controlled Register of Trials; HTA, Health Technology Assessment; NHS EED, National Health Service Economic Evaluation Database.
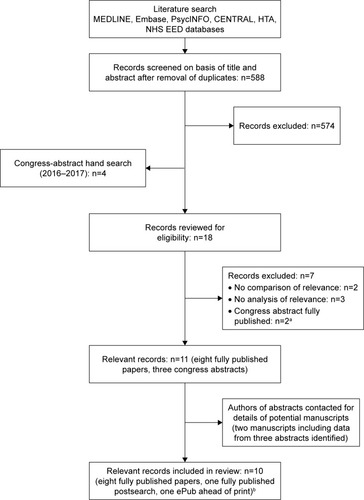
Table 1 Summary of studies comparing different GLP1 RAs included in the review
Table 2 Summary of studies comparing GLP1 RAs and insulin glargine included in the review
Figure 2 Frequency of individual treatment-attribute evaluation across ten patient-preference studies identified by literature review.
Abbreviations: AE, adverse event; BG, blood glucose; GI, gastrointestinal; HbAlc, glycated hemoglobin; MUP, multiuse pen; SBP, systolic blood pressure; SUP, single-use pen.
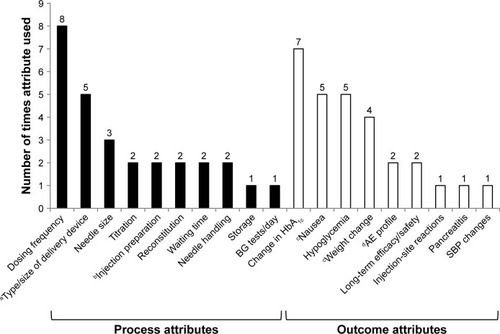
Table 3 Key results from DCEs evaluating preference for profiles of different GLP1 RAs in patients with T2DM
Table 4 Importance of attributes of GLP1 RA treatments determined in DCEs conducted in patients with T2DM
Figure 3 Preference for hypothetical GLP1 RA drug profiles determined in DCEs among patients with T2DM. (A) Preferences for a dulaglutide QW versus liraglutide QD profile among injectable-naive patients; (B) preferences for a exenatide QW versus liraglutide QD profile among injectable-naive or injectable-experienced patients; (C) preferences for a exenatide BID versus liraglutide QD profile in injectable and naive patients.
Abbreviations: BID, bis in die (twice daily); DCEs, discrete-choice experiments; QD, quaque die (once daily); QW, once weekly; RA, receptor agonist; SUP, single-use pen; T2DM, type 2 diabetes mellitus.
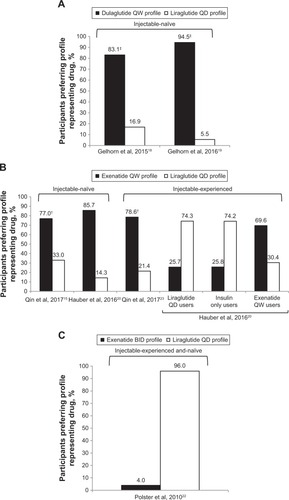
Table 5 Health-utility scores for health states described by different attributes of injection-delivery systems for weekly GLP1 RA therapies in patients with T2DM in UK and Italian TTO evaluations
Table 6 Overview of key results from studies that compared preferences for treatment attributes of GLP1 RAs with insulin glargine
