Figures & data
Table 1 Summary of the Most Relevant Studies Examining Transdermal Asenapine
Table 2 The Inhibitory Constant of Asenapine and Potential Neurotransmitters
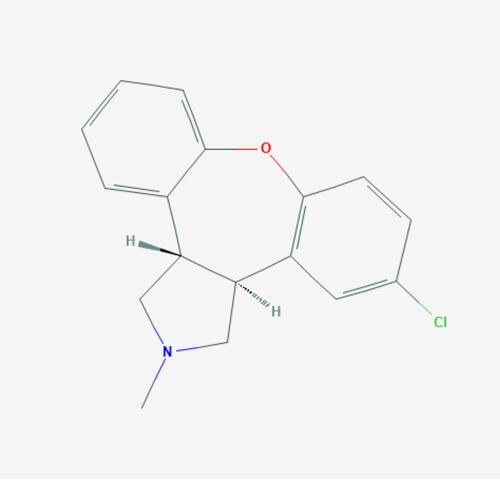
Figure 2 Change in PANSS score in subjects with acute schizophrenia and baseline score of 97.4 (placebo), 97.0 (low dose), and 95.6 (high dose). Data From Citrome et al’s study.Citation53
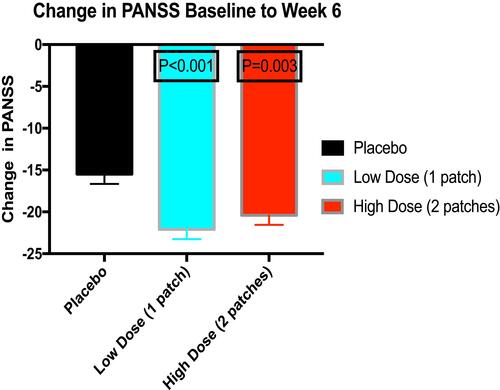
Figure 3 Response rates over time in in subjects with acute schizophrenia treated with low-dose transdermal asenapine (3.8 mg/24 hours), high-dose transdermal asenapine (7.6 mg/24 hours), and placebo. Only week 6 is significantly higher than placebo for both doses (P < 0.05). Data from Citrome et al’s study.Citation3
Table 3 Treatment-Emergent Adverse Effects (TEAE) That Occurred in the Phase 3 Trial of Transdermal Asenapine at a Rate of 5% of More in Subjects Receiving at Least One Dose