Figures & data
Table 1 User Population in the Study
Table 2 Target and Actual Cap-Removal Force for Non-Functional Autoinjector Devices in This Study
Figure 1 Non-functional mock-up autoinjector device used to simulate decapping handling step before (A.1) and after cap removal (A.2), and fully functional pre-filled autoinjector device before (B.1) and after cap removal (B.2).
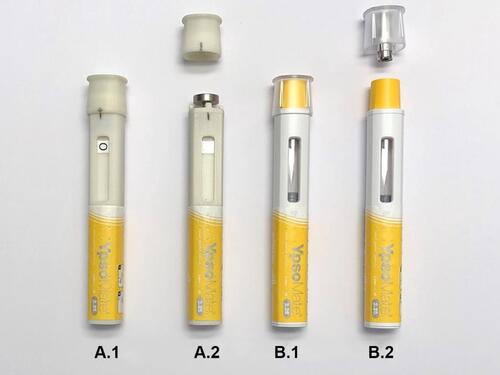
Table 3 Participant Ability to Decap Autoinjector Devices (A) and User-Reported Perceived Ease of Decapping Measured Using a 5-Point Likert Scale (B) per User Group and Device Type (FT25–FT55)
Figure 2 Participant-rated perceived ease of decapping using a 5-point Likert scale per autoinjector device type with target autoinjector-cap removal forces between 25 N and 55 N.
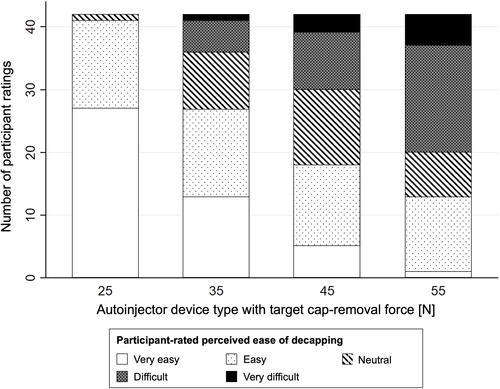
Table 4 Summary Statistics for the Linear Regression Model Considering Ease of Decapping as Response Variable
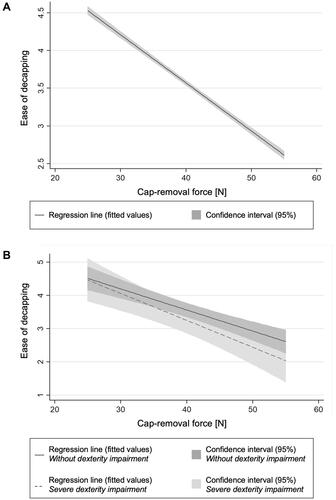
Figure 3 Estimated linear relation (pooled OLS model) between autoinjector-cap removal force and perceived ease of decapping for all participants (A) and for participants with severe dexterity impairment (M-SACRAH ≥ 5.0) and for participants without dexterity impairments (M-SACRAH = 0.0) (B).